A novel disorder of sex development, characterized by progressive regression of testicular function and cystic leukoencephalopathy
Abstract
We report a novel syndromic disorder of sex development observed in three male siblings, presenting with the association of micropenis without hypospadias, cryptorchidism, very low level of antimüllerian hormone in the neonatal period, and no persistent müllerian duct structures, suggesting a progressive regression of testicular function. The patients described here showed a striking neurological involvement including bilateral periventricular cysts observed in the anterior part of the frontal horns prenatally and increasing in size and number over time, associated with infra and supratentorial parenchymal atrophy, dilated ventricular system, corpus callosum hypoplasia, severe intellectual disability, and epilepsy. Associated features included a distinctive facies, joint contractures, retinopathy, and hearing loss. Pathological examination was consistent with testicular dysgenesis and leukoencephalopathy with spongiosis and microcalcifications. To the best of our knowledge, this disease, characterized by a recognizable pattern of malformations, has not been previously reported. An exhaustive genetic and metabolic evaluation was normal. Autosomal recessive inheritance was considered to be likely, on the basis of SNP studies. We hope that the detailed description provided here of the clinical, radiological, and pathological findings observed in this family will help to identify further unrelated patients, and ultimately, to clarify the genetic basis of this condition. © 2017 Wiley Periodicals, Inc.
INTRODUCTION
Disorders of sex development (DSD) include all congenital conditions in which development of chromosomal, gonadal, or anatomic sex is atypical [Arboleda et al., 2014]. DSD include a wide spectrum of developmental abnormalities ranging from isolated hypospadias to ambiguous genitalia. Overall, the incidence of DSD is estimated at about 1 in 1,000 live births [Arboleda et al., 2013; Ostrer, 2014]; the severe end of the spectrum, 46,XY gonadal dysgenesis, caused by mutations in genes involved in testicular development, is estimated to occur in less than 1 in 10,000 live births.
The etiology of DSD has been only partially elucidated. The availability of chromosomal microarray (CMA) and whole exome sequencing (WES) has led to the characterization of the genetic bases of an increasing number of DSD with defects in hormonal function [Arboleda et al., 2014; Ostrer, 2014]. Nevertheless, the molecular bases of many non-hormonal morphological abnormalities affecting genitalia are still unknown [Hutson et al., 2014]. Overall, only a minority of individuals affected by DSD currently obtains a genetic diagnosis, making it difficult to provide appropriate genetic counseling [Arboleda et al., 2013, 2014; Hutson et al., 2014; Ostrer, 2014]. Indeed, concerning rare DSD, if copy number variations (CNV), or single-nucleotide variants (SNV) are found in a single family, it is often difficult to formally prove their pathogenic role, especially when it is not possible to test further unrelated patients showing similar phenotypes. In these cases, detailed clinical reports can help to identify further affected patients, and ultimately, to clarify the genetic basis of these conditions.
We report a novel syndromic DSD observed in three male siblings and characterized by the association of gonadal dysgenesis with a progressive regression of testicular function and cystic leukoencephalopathy.
CLINICAL REPORT
Patient 1
This boy was the first child of healthy non-consanguineous parents. At conception, the mother was 27 years old and the father was 28 years old. Family history was unremarkable.
Pregnancy was characterized by intrauterine growth restriction (IUGR) observed at 32 weeks of gestation (WG). Delivery occurred at 38 WG. At birth, weight was 2,620 g (−2 SD); length was 48 cm (−1 SD); and OFC was 31 cm (−3,5 SD). Apgar score was 6 and 7 at 1 and 5 mins, respectively. A DSD was noted at birth including micropenis (length about 0.5 cm) without hypospadias and impalpable testes. Endocrinological assessment showed very low plasma levels of antimüllerian hormone (AMH) and testosterone and raised levels of FSH for age (Table I). Human chorionic gonadotropin (HCG) stimulation test failed to induce a rise in testosterone level, consistent with a congenital absence of functional testicular tissue. Androgen treatment was performed in order to improve penis length, with partial efficacy.
| 1 d | 28 d | 5 y | 6 y | 8 y | Normal values | |
|---|---|---|---|---|---|---|
| AMH (pmol/L) | 2 | 33 | np | <1 | <1 | 1,044 ± 441 (498–2,352) (prepubertal) |
| Testosterone (nmol/L) | 0.38 | 0.2 | np | <0.2 | <0.2 | 1–2 m: 8.81 ± 2.7 >2 m/prepubertal: 0.28 ± 0.1 |
| LH (IU/L) | np | 0.86 | np | np | 15 | 1 m: >1 8 y: <0.8 |
| FSH (IU/L) | np | 13.90 | np | np | 75 | <8 |
| DHAS (nmol/L) | np | np | 1,581 | 1,360 | 2,627 | 384 ± 61 |
- d, days; m, months; y, years; np, not performed.
- The patient also showed persistently raised levels of DHAS.
Severe developmental delay was noted when the child was able to hold his head at 20 months, but never reached the sitting position. Speech was absent. There were neither sleep nor behavioral problems. At 5 months he had generalized seizures clinically characterized by hypertonia and staring spells, which improved with valproate treatment.
He was referred to our department at 26 months. OFC was 46.4 cm (−2.5 SD). Dysmorphic features (Fig. 1A–C) included narrow palpebral fissures, strabismus and nystagmus, apparent widely spaced eyes, malar hypoplasia, prognathism, and a large mouth. Genital examination showed micropenis, no palpable testicles, and some sparse pubic hair. Ankles contractures and equinovarus feet were noted.
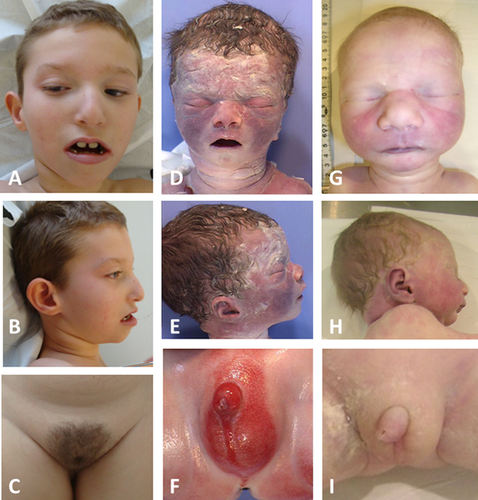
He developed scoliosis beginning at age 3 years. The foot deformity was treated by botulin toxin injections. At 4 years 9 months, OFC was 47.7 (−3 SD), stature was 112.7 cm (+1.5 SD), and weight was 20 kg (90th centile). He had multiple joint contractures (possibly secondary to the neurological impairment), camptodactyly of the fifth fingers, and normally ossified patellae.
Premature pubarche was confirmed, which was not explained by the androgenic treatment administered in the first months of life. At 5 years 10 months pubic hair was consistent with P4 stage of the Tanner sexual maturity rating system, and further endocrinologic evaluation showed advanced bone age (estimated at 8 years). Blood hormonal tests confirmed persistently low levels of AMH and testosterone, raised levels of FSH and DHAS for age (Table I). Computer tomography (CT) did not show adrenal hypertrophy.
He suffered from recurrent urinary infections. Abdominal ultrasounds scan (USS) and cysto-genitography showed an enlarged prostatic utricle and a small paravertebral spleen, testes were not found. Repeated echocardiographies were normal.
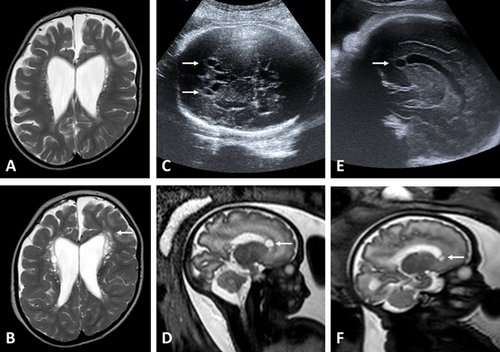
Repeated brain Magnetic Resonance Imaging (MRI) performed at 6 months, 2 years and 2 months, 4 years, and 7 years showed periventricular cysts which increased in size and number over time, progressive leukoencephalopathy, and generalized brain atrophy with corpus callosum hypoplasia (Fig. 2A and B). Magnetic Resonance Spectroscopy was normal and CT showed neither calcification nor other significant anomalies.
Fundus oculi examination was normal, except mildly pale optic discs. Repeated electroretinograms showed an abnormal pattern with reduced b-wave, consistent with a retinopathy. Visual evoked potentials did not show significant anomaly. Auditory evoked potentials showed sensorineural hearing loss.
The child unfortunately died at age 8 years 9 months old because of aspiration pneumonia; autopsy was not performed. A possible recurrence risk of 25% was reported to the couple.
Patient 2
The second pregnancy was characterized by a recurrence. Ultrasound evaluations, performed at 17 and 22 WG, were normal; the fetus was a male. Ultrasound performed at 32 WG showed incomplete development of male genitalia, including cryptorchidism and micropenis, and frontal bilateral paraventricular cysts (Fig. 2C). Fetal MRI confirmed these findings (Fig. 2D). Standard karyotype was normal. A termination of pregnancy (TOP) was performed at 34 WG + 6 days.
The biometric values corresponded to a fetus of 36 WG. The fetus weight was 2,265 g (−0.3 SD); crown-to-heel length was 49.5 cm (+0.7 SD); crown-to-rump length was 34.5 cm (+0.6 SD); and OFC was 34.5 cm (+1.4 SD). Physical examination revealed apparent widely spaced eyes, no palpable testis, and a short genital tubercle (inferior length: 5 mm; superior length: 7 mm) without hypospadias (Fig. 1D–F). Radiographs showed mild calcaneal stippling.
On macroscopic examination, atrophic testicles were found in the left inguinal canal and in the abdomen. Histological examination showed prostatic tissue, seminal vesicles, deferent ducts, and prostatic utricle. Cavernous body and corpus spongiosum were found in the genital tubercle. No persistent Müllerian structures were identified. Both testes were hypoplastic and histologically characterized by gonadal dysplasia (Fig. 3). The tunica albuginea was thin, resembling ovarian stroma. This peripheral zone contained abnormal epithelial nests or cords resembling seminiferous tubules together with larger cells corresponding to germ cells. The tubules and the germ cells were disorganized with some germ cells being isolated within the stroma. The central part contained a reduced number of seminiferous tubules. Testicular lobules were separated by unusually dense fibrotic interstitial tissue. Immunohistochemical analysis showed a reduced number of all testicular main cells (Sertoli, Leydig, and germ cells). This histopathological pattern is classically referred to as testis dysgenesis.
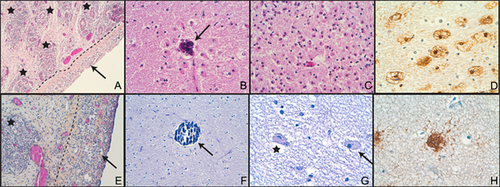
Neuropathological examination was limited by autolysis. Macroscopically, brain weight, and gyration were normal. The corpus callosum was thin. Pathological lesions were confined to the white matter (Fig. 3B–D) showing a soft appearance, especially in the frontal lobes and in the atrium region. Microscopically, this softening corresponded to lesions of leukoencephalopathy with perivascular microcalcifications (Fig. 3B), diffuse astrogliosis (Fig. 3C and D), and small areas of cavitation. Given the poor preservation of the brain, it was impossible to conclusively confirm the presence of the two frontal anterior periventricular cysts observed by MRI. The cortex was normal. No cerebellar or brain stem abnormalities were identified. The placenta was histologically normal.
Patient 3
The third pregnancy was unfortunately characterized by a further recurrence, diagnosed at the USS and MRI performed at 33 WG (previous USS were normal). This boy had bilateral frontal paraventricular cysts, similar to his brother (Fig. 2E and F), but no genital abnormalities were noted prenatally. TOP was performed at 35 WG + 2 days.
The biometric values corresponded to 36 WG. His weight was 2,550 g (+0.4 SD); crown-to-heel length was 48 cm (+0.1 SD); crown-to-rump length was 31.5 cm (−1 SD); and OFC was 32.5 cm (+0.3 SD). Physical examination revealed widely spaced eyes and low-set ears. The penis length appeared to be normal (13 mm) and testicles were found at the end of the inguinal canals.
Macroscopic examination revealed mild right ureteral dilatation (0.5 cm) and incomplete lung lobation. On histological examination, both testes showed the same lesions of gonadal dysplasia as previously described for patient 2 (Fig. 3E).
Brain weight and gyration were normal. Neuropathological examination confirmed the presence of two symmetrical paraventricular pseudocysts located at the anterior part of the frontal horns. Histologically, these pseudocysts did not correspond to subependymal pseudocysts or periventricular leukomalacia, but resulted from a gap between the ventricular wall and the deep white matter. The corpus callosum was thin. Histologically, diffuse lesions of the white matter were observed, including perivascular microcalcifications (Fig. 3F), astrogliosis, and micro-spongiosis (Fig. 3G). Compared with patient 2, astrogliosis was less marked. Astrocytes had a vacuolated cytoplasm (Fig. 3G and H). Some had a “Creutzfeldt cells” appearance (Fig. 3G), characterized by multiple micronuclei or chromatin clumps and large cytoplasm, representing an unusual form of reactive astrocytosis. Cortex was normal. No cerebellar or brain stem abnormalities were identified.
Metabolic and Genetic Evaluation
Complex genetic evaluation performed in patient 1 was normal including standard karyotype, CMA (105k, Agilent®), molecular analysis of SRY, SOX9, WT1, SF1, ARX, and exon 18 of KAT6B; hemoglobin electrophoresis, blood smear (absence of Heinz bodies), and molecular analysis of ATRX; maternal pattern of X inactivation.
Metabolic assessment was normal including plasma levels of cholesterol precursors; plasma levels of very long chain fatty acids, phytanic acid, pipecolic acid; lignocerate peroxisomal oxidation analysis and dihydroxyacetone-phosphate acyltransferase enzymatic assay performed on cultured fibroblasts; screening for congenital disorders of glycosylation; plasma ammonia; repeated plasma amino acids; urine amino acids; screening for creatine metabolism and adenylosuccinate lyase deficiencies. Urine organic acids, lactic acid levels in blood, and cerebrospinal fluid, molecular analysis of the mitochondrial mutations A3243G, A8344G, T8993G, and T8993C were normal, as were pyruvate dehydrogenase and pyruvate carboxylase enzymatic assays in fibroblasts.
An X-linked pattern of inheritance was hypothesized: genotyping analyses, performed using Illumina Infinium Human Core 12v1-0 chip (Integragen company), showed that the three patients did not share a region on the X chromosome (data not shown), thus ruling out this hypothesis.
This familial observation was submitted to the Dyscerne network [Krystyna et al., 2010] but no other diagnostic hypothesis was suggested.
DISCUSSION
We report the clinical, pathological, and molecular study of three brothers affected by a previously undescribed syndromic DSD. The main features are reported in Table II.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age of examination | 7 y 7 m | 34 WG and 6 d | 35 WG |
| Genital abnormalities | |||
| Micropenis | + | + | — |
| Hypospadias | — | — | — |
| Cryptorchidism | + | + | + |
| Testicular dysgenesis | + | + | + |
| Persistent müllerian structures | — | — | — |
| Premature pubarche | + | NA | NA |
| CNS abnormalities | |||
| Periventricular cysts | + | + (frontal) | + (frontal) |
| Leukoencephalopathy | + | + | + |
| Epilepsy | + | NA | NA |
| Severe intellectual disability | + | NA | NA |
| Facial features | |||
| Microcephaly | + | — | — |
| Mild widely spaced eyes | + | + | + |
| Narrow palpebral fissures | + | — | — |
| Strabismus | + | NA | NA |
| Nystagmus | + | NA | NA |
| Large mouth | + | — | + |
| Low set ears | + | + | + |
| Beaked nose | + | — | — |
| Skeletal abnormalities | |||
| Multiple joint contractures | + | — | — |
| Scoliosis | + | — | — |
| Other | Retinopathy hearing loss | Mild calcaneus stippling | Mild right ureteral dilatation, incomplete lung lobation |
- y, years; m, months; WG, weeks of gestation; d, days; +, present; −, absent; NA, not applicable; CNS, central nervous system.
During human embryogenesis, sex development occurs in sequential stages. First, at the end of the 4th week of gestational age, the adrenogonadal primordium deriving from the urogenital ridges divides in two different structures giving rise to the gonads and the adrenal glands. Second, during the sex determination stage, the bipotential gonads differentiate to either male or female gonads [Arboleda et al., 2014]. SRY, located on the Y chromosome, acts as a dominant male determinant. At approximately 7 weeks after conception, upregulated SRY expression stimulates, in Sertoli cells, a cascade of gene expression including the Sry-box-containing gene 9 (SOX9) [Arboleda et al., 2014], which induces the secretion of AMH. Subsequently, in the sex differentiation stage, AMH determines the regression of the Müllerian ducts, which are the precursors of female genital structures (Fallopian tubes, uterus, and vagina) while testosterone and dihydrotestosterone, secreted by Leydig cells, stimulate the development of male internal and external genital organs (prostate, vas deferens, penis, and scrotum) [Arboleda et al., 2014]. The DSD observed in the three siblings here reported is very unusual. The presence of micropenis without hypospadias, the very low levels of AMH detected in the neonatal period and the absence of persistent Müllerian derived structures, are consistent with a gonadal dysgenesis characterized by the presence of a testicular function in the early stages of pregnancy (until 16–18 WG), with a subsequent regression.
Brain abnormalities were also striking: prenatally, patients 2 and 3 presented with frontal periventricular cysts. The number and the size of cysts increased over time in patient 1 and white matter changes appeared progressively. On the basis of genotyping analysis, autosomal recessive inheritance was considered to be most likely, even if we cannot formally rule out germinal mosaicism of a dominant mutation.
The association between a DSD characterized by micropenis without hypospadias and regression of testicular function, and neurological involvement has been occasionally reported. Anderson et al. [2011] reported a male infant showing severe genital abnormalities including anorchia, micropenis, regression of penile corporeal tissue, absence or müllerian structures, associated with severe neurological involvement including a marked pontocerebellar hypoplasia (PCH) with virtually absent cerebellar hemispheres, hypotonia, seizures, and early death. Three boys (including two brothers) have been reported presenting with micropenis with normal penile structure, small cryptorchid testes at birth with subsequent regression, low basal LH and testosterone levels, absence of müllerian structures, and mild developmental delay [Parisi et al., 2002]; brain MRI performed in one patient showed some patchy areas of abnormal myelination with no other significant anomaly. In spite of some similarities concerning genital abnormalities, the three siblings here reported have significantly different neurological involvement, as compared with the previously reported patients (absence of PCH; presence of periventricular cysts; severe ID); moreover, retinopathy and hearing loss were noted in patient 1. To the best of our knowledge, this condition has not been described previously.
Further molecular analyses using next-generation sequencing are currently ongoing, but their interpretation will require performing these analyses in additional unrelated affected patients. This prompted us to provide a detailed description of the phenotype of this family, in the aim of helping clinicians to identify similar patients.
In conclusion, we report a novel syndromic DSD, characterized by a recognizable pattern of brain and genital malformations. We hope that this report will help to diagnose other affected unrelated patients, in order to further delineate the phenotypic spectrum of this condition, possibly also in female patients, and ultimately, to characterize its molecular basis.
ACKNOWLEDGMENTS
The authors are very grateful to patients’ family. We would like to thank Dr. Isabelle Rouvet, Centre de biotechnologie cellulaire, Hospices Civils de Lyon, Bron, France, for her precious collaboration, and Dr Antoinette Bernabe Gelot, Neuropathologie, Anatomie pathologique, Hôpital Trousseau, AP-HP, Paris, France, for her experienced opinion concerning patients 2.