Prenatal diagnosis of inverted duplication deletion 8p syndrome mimicking trisomy 18
Abstract
Inverted duplication deletion of 8p (invdupdel[8p]) is a well-described and uncommon chromosomal rearrangement. The majority of the reported cases have revealed no life-threatening malformations. Although the invdupdel[8p] syndrome in children with central nervous system abnormalities has been reported before, we present the first prenatal microarray diagnosis of invdupdel[8p] syndrome mimicking trisomy 18 due to similar sonographic features. Contrary to reported cases with invdupdel[8p] syndrome, the present case had severe polyvalvular dysplasia and the infant deceased at day 12 of life. In this case, we also emphasize the diagnostic power of microarray analysis in detecting the underlying genetic causes for fetuses with multiple congenital anomalies. © 2017 Wiley Periodicals, Inc.
INTRODUCTION
Inverted duplication deletion of 8p (invdupdel[8p]) is a chromosomal rearrangement and Weleber et al. [1976] presented the first case in 1976. Most of these cases are diagnosed in the childhood period due to neurodevelopmental delay. Here, we present a prenatal diagnosis of invdupdel[8p] at 31 weeks’ gestation with microarray analysis that was implemented after normal fluorescence in situ hybridization (FISH) results. We also purposed to highlight the increasing rate of the genetic factors as an underlying cause in the cases with congenital heart defects with or without extracardiac abnormalities.
CLINICAL REPORT
A 38-year-old, African American, gravida 3, para 1, woman was referred to our center for advanced fetal care at 31 weeks of gestation for an evaluation of a suspected congenital heart defect (CHD). Her obstetric history was unremarkable and the couple was nonconsanguineous.
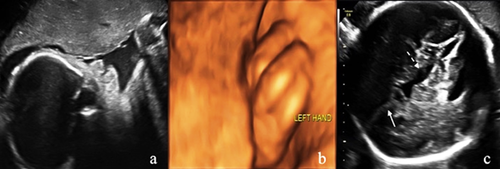
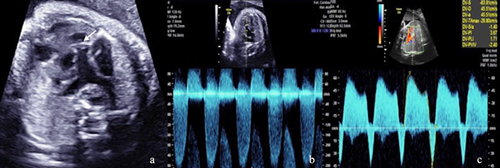
Ultrasonography revealed a fetus small for its gestational age with increased amniotic fluid volume (amniotic fluid index was 25.8 cm, with a maximum deepest pocket of 8.3 cm). In addition, both an absent nasal bone and clenched left hand (Fig. 1a,b) were demonstrated. Neurosonography showed agenesis of the corpus callosum, cerebellar hypoplasia (transcerebellar diameter <5th centile [p.] for 31 + 2 weeks), and enlarged third ventricle (7 mm × 2.3 mm) (Fig. 1c). Fetal echocardiography revealed enlarged thickened heart walls along with polyvalvular dyplasia (thickened tricuspid, aortic, and pulmonary valves) with regurgitation (Doppler flow for tricuspid, aortic, and pulmonary valves were 200, 170, and 220 cm/sec, respectively) (Fig. 2a). In addition, the main pulmonary artery and branches were dilated (Z score for MPA, LPA, and RPA were 6.11, 3.63, and 3.52, respectively) (Fig. 2b). Doppler examination showed elevated umbilical artery indices (pulsatility index [PI]: 1.4 and resistance index: 0.88, both were >95th centile.), brain sparing (middle cerebral artery PI: 1.52 <5th p.), and reversed a-wave flow in the ductus venosus (Fig. 2c). All sonographic findings suggested a complex cardiovascular disorder including but not limited to deficiency of forward cardiac output and possible hypotension due to poor myocardial function.
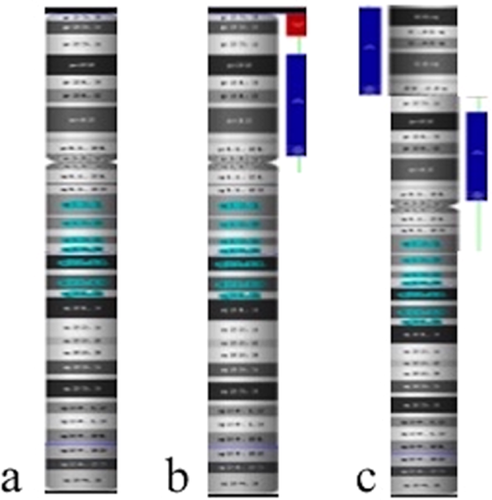
The family was provided with extensive genetic counseling regarding the strong association between the constellation of the ultrasound findings and chromosomal abnormalities. The couple elected to proceed with amniocentesis. Prenatal aneuploidy FISH was normal for a female fetus. Direct single nucleotide polymorphism (SNP) microarray analysis revealed a terminal deletion of 6.84 Mb, from 8pter to 8p23.1 (158.048–6.999.114) and a duplication of 31.22 Mb, from 8p11.21 to 8p23.1 (12.552.775–43.780.262). This result was consistent with the inverted duplication/deletion 8p syndrome (Fig. 3). There was an approximate 5 Mb normal copy number segment between the deleted and duplicated segments. The deleted and duplicated intervals included numerous OMIM genes.
After multidisciplinary team counseling including genetics, perinatology, pediatric cardiology, and neonatology about the diagnosis and range of prognoses, the family elected to continue the pregnancy. The patient had a vaginal delivery at 34 weeks’ gestation due to preterm premature rupture of membranes. The female fetus was born with a birthweight of 1840 g (<10. centile) and Apgar scores were eight and nine at 1 and 5 min, respectively. The postnatal echocardiography and neurosonography confirmed the prenatal findings. Additionally, postnatal echo showed muscular VSD and severe right ventricle hypertrophy. Metabolic acidosis and base deficit were observed after birth. Sodium carbonate and dopamine was started and continued for the rest of the patient's life without success. On the 4th day of life, furosemide was started for high O2 saturations and concern for pulmonary over-circulation. At 8 days of life, furosemide increased in response to pulmonary edema. On the 11th day of life, the infant developed necrotizing enterocolitis followed by worsened metabolic acidosis, base deficit, and poor perfusion. The critical and rapidly declining status of the baby was discussed with the parents and they requested for the withdrawal of care and extubation. The parents declined postmortem autopsy.
DISCUSSION
Invdupdel[8p] is a well-described and uncommon chromosomal rearrangement with an incidence rate of around 1 in 10,000–30,000 liveborn infants [García-Santiago et al., 2015]. In this case, we present the first known prenatal diagnosis of an invdupdel[8p] syndrome complicated with polyvalvuvar dysplasia and central nervous system (CNS) abnormalities.
Duplications of some part of the olfactory receptor (OR) gene clusters serve as a source for the rearrangements of the short arm of chromosome 8 [Giglio et al., 2001; Buysse et al., 2009]. The 8p23.1 region and the GATA4 gene are “hotspots” for fetal heart development, as deletion and duplication of 8p23.1 are associated with heart defects including: atrial and/or ventricular septal defects, double outlet right ventricle, dextrocardia, pulmonary stenosis, and hypoplastic left heart syndrome [Pehlivan et al., 1999; Keitges et al., 2013]. Large deletions of 8p23.1 including the GATA4 gene are associated with more severe CHD than heterozygous GATA4 point mutations [Wat et al., 2009]. In the present case, the deletion/duplication did not include the GATA4 gene; however, the duplication size was one of the largest that has ever been reported. Two previous studies reported a correlation between increased duplication length and severity of cognitive deficits [Fisch et al., 2011; García-Santiago et al., 2015]. In this case, we can speculate that large duplication length may be associated with CHD that was uncharacteristically severe for this disorder.
It is already known that in the inverted duplication/deletion 8p syndrome, structural abnormalities manifest, in descending order of frequency: CNS malformations (including agenesis of the corpus callosum), skeletal abnormalities, hypotonia, and congenital heart defects [García-Santiago et al., 2015]. In literature, about 50 cases of invdupdel[8p] have been reported in the postnatal period [Sireteanu et al., 2013]. The majority of these cases were diagnosed during childhood after identification of postnatal neurodevelopmental findings including speech delay, mental retardation, facial dysmorphism, and relatively mild CHD [Yenamandra et al., 1999; Fisch et al., 2011; García-Santiago et al., 2015]. Approximately 25% of cases of invdupdel[8p] syndrome possess an associated heart defect, most of which are not life threatening [Masuda et al., 2002; García-Santiago et al., 2015]. Macmillin et al. [2000] reported prenatal diagnosis of inverted duplication of 8p in two cases and the sonographic findings existed at 16 and 30 weeks gestation, respectively. Similar to our case, the latter one had agenesis of corpus callosum, ventriculomegaly, Dandy–Walker variant, and both atrial and ventricular septal defects along with depressed left ventricle function. Due to the lack of postnatal follow-up, we did not know whether the infant also deceased in the post-partum period due to circulatory failure. In our case, polyvalvular dysplasia and CNS anomalies were remarkable findings which were seen regularly in an autopsy series of trisomy 18 cases [Balderston et al., 1990]. The genetic test was ordered due to the strong association between these prenatal findings and trisomy 18.
Sireteanu et al. [2013] diagnosed invdupdel[8p] in a 5-year-old girl via conventional cytogenetic analysis followed by SNP. An additional material on chromosome 8 was identified by standard G-banding analyses. The authors proceeded with a SNP array and detected both a terminal deletion (8p23.3–8p23.1) and a duplication (8p23.1–8p11.1). In our case, we followed the same genetic approach and microarray analysis was performed after normal FISH result. We also emphasize the importance of prenatal diagnosis in the multidisciplinary setting for rare genetic rearrangements as mentioned previously [Sireteanu et al., 2013]. Our recommended approach in cases where multiple fetal anomalies are identified on ultrasound would be to first order prenatal aneuploidy FISH. If FISH is abnormal, we would suggest the next step be routine karyotyping to confirm the FISH. This would be done in order to exclude mosaicism and to identify whether the trisomy is the result of a translocation, which would require parental chromosome analysis for recurrence risk estimation. If the aneuploidy FISH is normal, we would then recommend direct SNP microarray. This technology would allow for the detection of microdeletions and microduplications, which will allow for the provision of proper counseling and prognostication during pregnancy.