Atypical Angelman syndrome due to a mosaic imprinting defect: Case reports and review of the literature
Abstract
Angelman syndrome (AS) is characterized by severe intellectual disability, limited, or absent speech and a generally happy demeanor. The four known etiological mechanisms; deletions, uniparental disomy, imprinting defects, and UBE3A mutation all affect expression of the UBE3A gene at 15q11-q13. An atypical phenotype is seen in individuals who are mosaic for a chromosome 15q11-q13 imprinting defect on the maternal allele. These patients present with a milder phenotype, often with hyperphagia and obesity or non-specific intellectual disability. Unlike typical AS syndrome, they can have a vocabulary up to 100 words and speak in sentences. Ataxia and seizures may not be present, and the majority of individuals do not have microcephaly. Here we review the current literature and present three individuals with atypical AS caused by a mosaic imprinting defect to demonstrate why DNA methylation analysis at the SNRPN locus needs to be considered in a broader clinical context. © 2017 Wiley Periodicals, Inc.
INTRODUCTION
Angelman syndrome (AS) is a neurogenetic disorder present in one in 12,000 to one in 20,000 Live births. It is characterized by severe developmental delay, microcephaly, ataxia, speech impairment, seizures, frequent laughter, and hand flapping. Dysmorphic facial features include wide mouth, maxillary hypoplasia, and prognathia [Clayton-Smith and Laan, 2003]. The four known etiological mechanisms all cause loss of function of the UBE3A gene, a maternally expressed gene within chromosome region 15q11-q13, essential for fetal neurodevelopment [Buiting, 2010]. Most cases of AS are accounted for by interstitial 15q11-q13 deletions (∼80%), uniparental disomy (∼7%), and UBE3A mutations (∼10%). The remaining ∼3% have an imprinting defect, where aberrant imprinting causes the maternal chromosome to carry a paternal imprint, thereby silencing the UBE3A gene [Lossie et al., 2001; Buiting et al., 2003]. In ∼90% of such cases, this imprinting defect is a sporadic primary epimutation, while the remaining cases are due to an inherited or de novo deletion within the AS imprinting center [Lossie et al., 2001; Williams et al., 2010]. In 40–50% of non-deletion imprinting defects, a post-zygotic imprint maintenance error occurs after the initial cell division [Buiting, 2010]. This results in all the subsequent daughter cells losing their maternal 15q11-q13 (SNRPN) methylation pattern, and therefore, causes somatic mosaicism [Nazlican et al., 2004]. The investigation of these patients by DNA methylation analysis yields an abnormal methylation pattern with reduced methylation (hypomethylation) of the maternal allele [Gillessen-Kaesbach et al., 1999; Buiting et al., 2003; Nazlican et al., 2004; Camprubí et al., 2007].
We describe three children who are mosaic for a 15q11-q13 imprinting defect, highlighting the importance of considering this diagnosis in a child with an atypical presentation of AS.
CLINICAL REPORT
Patient 1
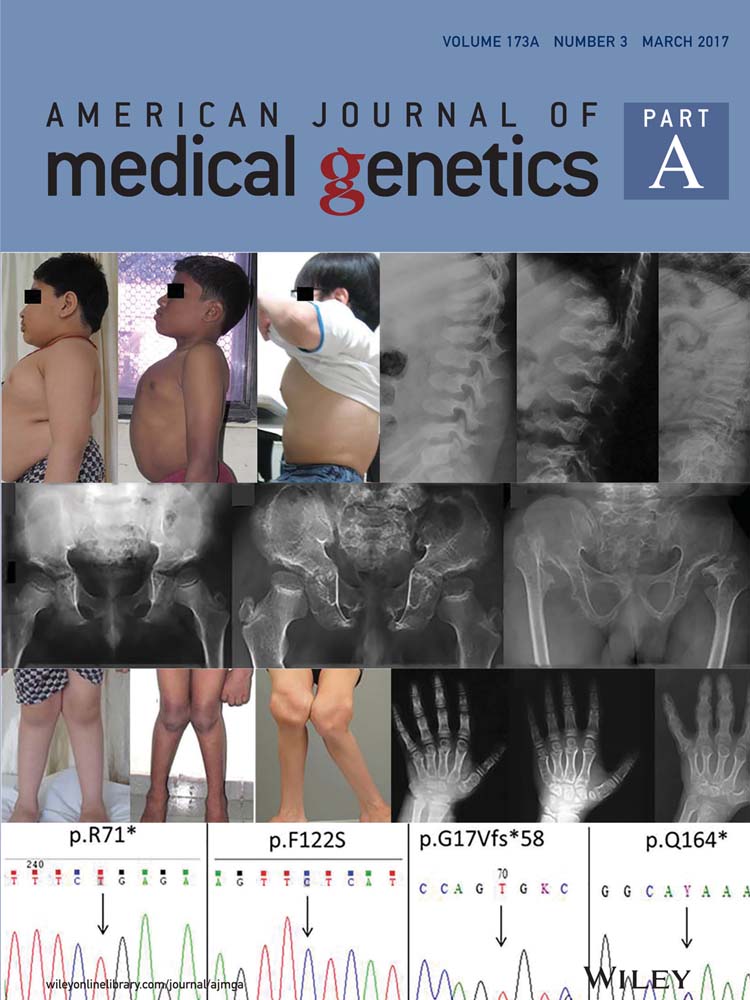
Patient 1 was a 17-year-old female born at full term with normal growth parameters to non-consanguineous parents. She sat at age 6 months, walked at 12 months, ran upstairs at 5 years and could ride a bicycle unassisted, with no history of ataxia. She spoke her first word at age 18 months and was using two-word phrases by age 5. At age 17, her vocabulary had over 100 words; she used simple three-to-four word sentences, could write her name and followed three-to-four step commands. She attended a special class for intellectually disabled children, and required help with hygiene. At age 17, she slept for 4 to 5 hours, but managed to stay in her bed all night. Her overall demeanor was happy and well-behaved, but she experienced episodes of aggression. She had no history of seizures. At age 17, her weight was 84 kg (90–97th centile), height 160 cm (25–50th centile), and OFC 54 cm (2nd–50th centile). Her BMI was 32.8 kg/m2 (>97th centile). Clinical features included bilateral clinodactyly, deep-set eyes, midface retrusion, and a large tongue. She had a wide mouth with the distance between the oral commissures at rest measuring 6.5 cm (> + 2SD) (Fig. 1). Cerebral MRI, EEG, molecular karyotype and fragile X testing were normal.

Patient 2
Patient 2 was a 16-year-old female born at full term with normal growth parameters to non-consanguineous parents. She walked at age 14 months, but had no speech until age 30 months. At age 16, she used three-to-four word sentences but was unable to write or read. She was mildly ataxic with no seizures. Her parents’ main concern was her steadily increasing weight gain despite a controlled diet. Although a sociable happy girl, she experienced episodes of aggression. At age 16, her height was 178.5 cm (>97th centile), weight 99.2 kg (>97th centile), and she was obese with a BMI of 31.1 kg/m2 (> 95th centile). There was no microcephaly. Chromosome microarray showed a female karyotype with an 8p23.1-p23.2 duplication thought to be a benign variant.
Patient 3
Patient 3 was an 11-year-old boy, one of non-identical twins delivered at 35 weeks with normal growth parameters. He walked at 22 months with a broad-based gait and was speaking two words by 24 months. He had a happy disposition with no seizures. His height and weight were both above the 97th centile by age 4. At age 11, his intellectual disability was assessed as moderate with speech limited to single words. His OFC was 55 cm (75th centile), height 161 cm (97th centile), weight 81 kg (> 97th centile). He was obese with a BMI of 31.2 kg/m2 (> 95th centile) and had widely spaced teeth. Fragile X testing and molecular karyotype were normal.
MOLECULAR STUDIES
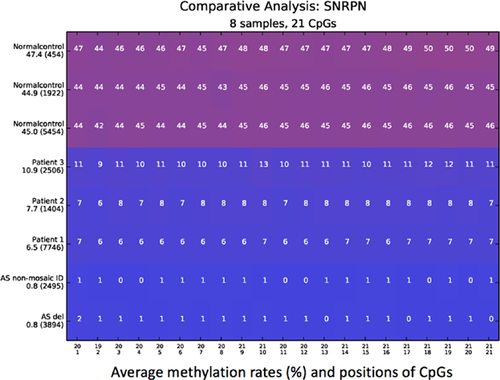
Methylation-specific (MS)-PCR of the 15q11-q13 region revealed a strong paternal unmethylated but a faint maternal methylated band in the three patients [Zeschnigk et al., 1997]. By methylation-specific Multiplex Ligation-Dependent Probe Amplification (MS-MLPA) (SALSA MLPA-kit ME028-B2, MRC Holland), hypomethylation of the methylation-specific SNRPN probes was observed, confirming the results of the MS-PCR. A large deletion of 15q11-q13 and a small deletion of the imprinting center was excluded by MLPA gene dosage analysis. Uniparental disomy 15 testing was negative, indicating that all three patients had a cellular mosaicism for a sporadic imprinting defect with a small number of methylated SNRPN alleles representing normally methylated cells. We performed highly quantitative next generation sequencing on bisulfite treated DNA using the Roche/454 Genome Sequencing junior system to quantify the degree of methylation. Locus-specific amplicon libraries for each individual were generated as described by Beygo et al. [2013] (PCR conditions and primer sequence are available by request). We analyzed 21 CpG dinucleotides in the exon1/promoter region of SNRPN. For the patients we analyzed 1400 up to 7700 single sequence reads, respectively. For this analysis, the Python-based amplikyzer software was used (Rahmann et al., Amplikyzer: Automated methylation analysis of amplicons from bisulfite flowgram sequencing. PeerJ PrePrints 1:e122v2 https://doi.org/10.7287/peerj.preprints.122v2''7/peerj.preprints.122v2).
Three normal controls and two patients with AS, one with a maternal deletion of 15q11-q13 and one with a non-mosaic imprinting defect, were included in the analysis. An average of 45–47.4% mean methylation in the three normal controls and an average of 0.8% for both AS control patients was observed. We found a similar degree of methylation in Patient 1 and 2 with a mean methylation of 6.5% and 7.7%, respectively. Patient 3 showed a slightly increased mean methylation of 10.9% (Fig. 2).
Multi-locus imprinting disturbances (MLID) have not been described in patients with AS but are a frequent finding in patients with other imprinting defects. We studied methylation for several other imprinted loci to exclude MLID in the three reported patients. We used MS-MLPA (SALSA MLPA-kits ME030, ME031, ME032-A1, ME034-A1, MRC Holland) for methylation analysis of the H19, KCNQ1OT, PLAGL1, MEST, GRB10, MEG3, PEG3, and GNAS loci. We found normal methylation levels at all loci studied and could thus rule out a MLID in the three patients (data not shown).
DISCUSSION
In most AS patients (except for those with a UBE3A mutation or unknown etiology), the typical 15q11-q13 methylation pattern is the complete absence of methylation due to the absence of a maternal allele (deletion or uniparental disomy) or the presence of a maternal allele with an incorrect paternal epigenotype (imprinting defect) [Buiting, 2010; Williams et al., 2010]. However, DNA methylation analysis of mosaic individuals has shown a reduced methylation compared to normal controls, indicating the presence of a mixture of normal cells and cells containing an AS imprinting defect on the maternal allele [Gillessen-Kaesbach et al., 1999; Brockmann et al., 2002; Nazlican et al., 2004; Lawson-Yuen et al., 2006; Camprubí et al., 2007; Fairbrother et al., 2015]. Table I summarizes the clinical features of our three patients along with the 28 previously published patients with confirmed mosaic Angelman syndrome [Gillessen-Kaesbach et al., 1999; Brockmann et al., 2002; Nazlican et al., 2004; Lawson-Yuen et al., 2006; Camprubí et al., 2007; Fairbrother et al., 2015].
| Total number of individuals with feature reported | Number in which feature is present (%) | Age (years) | |
|---|---|---|---|
| Speech | 26 | 3–17 | |
| No words | 4 (15.5) | ||
| <3 words | 4 (15.5) | ||
| 3–10 words | 6 (23) | ||
| >10 words | 12 (46) | ||
| Good comprehension skills | 21 | 13 (62) | |
| Average age of speech onset mean (range) | 19 | 2.6 (1–7) | |
| Average age of onset of walking unassisted mean (range) | 23 | 1.5 (1–3) | |
| Walked by age 5 | 24 | 24 (100) | |
| Ataxia | 25 | 11 (44) | |
| Seizures | 28 | 8 (29) | |
| Microcephaly | 26 | 3 (12) | |
| Hypopigmentation | 11 | 2 (18) | |
| Abnormal EEG | 11 | 7 (64) | |
| Obesity | 25 | 8 (32) | |
| Hyperphagia | 20 | 11 (55) | |
| Muscular hypotonia | 23 | 6 (26) | |
| Neonatal feeding problems | 24 | 7 (29) | |
| Behavioral problems | 5 | 4 (80) | |
| Sleeping difficulties | 6 | 5 (83) | |
| Inappropriate laughter | 24 | 9 (37) | |
| Happy disposition | 6 | 6 (100) | |
| Facial dysmorphic features | 9 | 3 (33) | |
| Referral diagnosis PWS | 22 | 11 (50) |
Although there is broad phenotypic overlap, patients with an interstitial deletion are most likely to have the classically described AS phenotype whereas individuals with a UBE3A mutation have an intermediate phenotype. In contrast, those with unparental disomy, an imprinting defect or mosaic AS tend to have a milder phenotype. These variable AS phenotypes are summarized in Table II [Varela et al., 2004; Tan et al., 2011; Horváth et al., 2013; Bai et al., 2014, Luk and Lo, 2016]. Importantly, the phenotype of individuals with mosaic AS may not be entirely consistent with the diagnostic features recommended by the 2005 Consensus Statement of the United States AS Foundation [Williams et al., 2006]. In particular, these atypical individuals have a greater ability to speak, with several documented cases of mosaic individuals being able to speak more than 10 words and form simple two-to-three word sentences. Our first patient was reported to have a vocabulary of more than 100 words, which is more than any other previously reported individual in the literature. They are also less likely to present with a movement or balance disorder. 100% of patients could walk unassisted by age 5, and Patient 1 learned to ride a bicycle. Moreover, patients with mosaic imprinting defects had a lower incidence of typical AS behavioral features, seizures, and microcephaly which may delay diagnosis. A BMI >80% centile was a common trait among these individuals; however, a clinical suspicion of Prader–Willi syndrome may have caused ascertainment bias [Gillessen-Kaesbach et al., 1999; Brockmann et al., 2002; Nazlican et al., 2004; Lawson-Yuen et al., 2006; Camprubí et al., 2007; Fairbrother et al., 2015].
| Mechanism | Interstitial deletion | UBE3A | UPD | Imprinting defect | Mosaic Angelman (n = 31) |
|---|---|---|---|---|---|
| Speech | n = 21 | n = 48 | n = 27 | n = 20 | n = 26 |
| Age (y) | 10.9 | 5.3–17.3 | 8.9 | 10.6 | 3–17 |
| No words | 71% | 33% | 44% | 40% | 15.5% |
| <3 words | 29% | 25% | 15% | 15% | 15.5% |
| 3–10 words | – | 42% | 33% | 30% | 23% |
| >10 words | – | – | 8% | 15% | 46% |
| Seizures | n = 202 | n = 53 | n = 42 | n = 20 | n = 28 |
| Age (y) | 2.7–10.9 | 2.7–17.3 | 1.3–9 | 11 | 3–17 |
| Seizures present | 83% | 68% | 40% | 40% | 29% |
| Body mass index | n = 57 | n = 21 | n = 24 | n = 16 | n = 25 |
| Age (y) | 4.8–10.9 | 11.2–17.3 | 1.3–8.9 | 11 | 3–17 |
| BMI >95% | 4% | 19% | 42% | 63% | 32% |
| HC | n = 201 | n = 53 | n = 43 | n = 21 | n = 26 |
| Age | 2.7–10.9 | 2.7–17.3 | 1.3–9 | 11 | 3–17 |
| HC <5% | 67% | 55% | 14% | 10% | 12% |
In a previous study, Nazlican et al. [2004] investigated a cohort of 24 mosaic individuals with different degrees of mosaicism ranging from ∼1% to 40% using a quantitative real-time DNA methylation assay. Regression analysis suggested that individuals with a higher percentage of normally methylated cells tend to have a milder phenotype, but for the sum of clinical features as well as for individual features the correlation was not statistically significant (P = 0.139). Based on the percentage of methylation, we would expect Patient 3 to have the mildest phenotype of our three patients, yet it was Patient 1 who had remarkably good language skills. Although we were unable to test skin from our patients, differences in the percentage of mosaicism within different tissues, most importantly in brain cells, could account for phenotypic variation in patients with mosaic AS.
The cause of intellectual disability remains unexplained in at least 50% of affected individuals [Willemsen and Kleefstra, 2014]. A number of these patients may show some signs of AS, while not having all of the classical features. Significantly greater receptive communication skills in comparison to the level of verbal skills in a child with developmental delay should prompt SNRPN DNA methylation testing for a potential Angelman imprinting defect mosaicism. This clinical suspicion should be further increased in the presence of any of the other physical or behavioral features of Angelman or Prader–Willi syndromes.
ACKNOWLEDGMENTS
We thank the patients and their families for their contribution to this work.