Analysis of copy number variants in 11 pairs of monozygotic twins with neurofibromatosis type 1
Abstract
Phenotypic variability among individuals with neurofibromatosis type 1 (NF1) has long been a challenge for clinicians and an enigma for researchers. Members of the same family and even identical twins with NF1 often demonstrate variable disease expression. Many mechanisms for this variability have been proposed. We have performed an exploratory study of copy number variants (CNVs) as a possible source of phenotypic variability in NF1. We enrolled 11 pairs of monozygotic (MZ) twins with NF1 and their parents, catalogued their clinical characteristics, and utilized a single nucleotide polymorphism (SNP) microarray to identify CNVs in blood and saliva. The 11 twin pairs showed high concordance for presence and number of café-au-lait spots, cutaneous neurofibromas, IQ, and ADHD. They were more likely to be discordant for optic pathway glioma, plexiform neurofibromas, skeletal manifestations, and malignancy. Microarray analysis identified a total of 81 CNVs meeting our conservative criteria, 37 of which overlap known genes. Of interest, three CNVs were previously unreported. Microarray analysis failed to ascertain any CNV differences within twin pairs, between twins and parents, or between tissues in any one individual. Results of this small pilot study did not demonstrate any de novo CNV events in our MZ twin pairs, nor were de novo CNVs overrepresented in these individuals with NF1. A much larger sample size would be needed to form any conclusions about the role of CNVs in NF1 variable expressivity. Alternative explanations for discordant phenotypes include epigenetic changes, smaller genetic alterations, or environmental factors. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Individuals with neurofibromatosis type 1 (NF1) are at an increased risk of developing both benign and malignant tumors, skeletal and vascular abnormalities and learning disabilities [Friedman and Birch, 1997]. One of the most challenging aspects of managing patients with NF1 is the extreme variability of phenotype. Currently, there is no way to predict which patients are at high risk for serious complications like malignancy, and to prospectively identify and screen those individuals. Despite extensive research, the underlying mechanism for this variability has never been elucidated. Second-hit events in the NF1 gene have been well-documented in the majority of cutaneous neurofibromas [Thomas et al., 2012] and at least one skeletal complication of NF1 [Stevenson et al., 2006], but are unlikely to explain the entire spectrum of NF1 features. Twin studies have historically been a valuable tool for studying genetic disorders. The literature reports at least 30 pairs of proven MZ twins with NF1 [Brady, 1962; Vaughn et al., 1981; Cartwright, 1982; Akesson et al., 1983; Crawford and Buckler, 1983; Bauer et al., 1988; Pascual-Castroviejo et al., 1988; Craigen and Clarke, 1995; Kelly et al., 1998; Tubridy et al., 2001; Koul et al., 2000; Payne et al., 2003; Lubinski, 2006; Sabbagh et al., 2009; Rieley et al., 2011]. In a population of 175 individuals from 48 families with NF1, Easton et al. [1993] found that phenotypic features varied to a greater degree with increasing distance from a proband, with six pairs of MZ twins having the closest agreement in traits such as presence of neurofibromas, head circumference and learning disabilities, compared to more distant relatives with NF1. They concluded that there was significant evidence for modifying loci affecting NF1 expression [Easton et al., 1993]. A similar larger French study of 750 NF1 patients from 275 families also provided evidence for genetic modifiers unlinked to the NF1 Locus [Sabbagh et al., 2009].
Although MZ twins were previously assumed to be genetically identical, recent studies have shown remarkable differences in MZ twins in areas such as copy number variants (CNVs) [Bruder et al., 2008]. Copy number variants are microduplications or deletions of DNA that are typically defined as being larger than one kilobase (kb), and are now known to be widespread throughout the human genome [Scherer et al., 2007]. There is mounting evidence that CNVs have played a significant role in normal population variation, evolution, and disease predisposition [Wong et al., 2007; McCarroll et al., 2008] and significant CNV associations have been found in several neuropsychological disorders [Sebat et al., 2007; Xu et al., 2008].
Some CNVs represent recurrent polymorphisms occurring at a low rate in the population and have no known clinical significance. However, more than 50% of CNVs occur in areas of known genes and have the potential to impact phenotype [Iafrate et al., 2004]. According to some reports, the average person would be expected to have 10–50 CNVs using current SNP-based microarrays, with the majority of those (>90%) being familial [Iafrate et al., 2004; Conrad et al., 2006; McCarroll et al., 2008].
In this study, we used a SNP microarray platform to analyze CNVs in 11 pairs of MZ twins with varying phenotypic concordances and discordances, in order to identify potential genetic factors which may influence disease manifestation. Secondarily, we explored the hypothesis that individuals with NF1 may have a higher incidence of de novo CNVs, due to deregulation of Ras-mediated cell cycle control mechanisms.
MATERIALS AND METHODS
Recruitment
Participants included 11 pairs of monozygotic twins with NF1 (ages 5–26 years) and their parents. Eight of these twin pairs had been previously included in a published study of variable expression of NF1 in twins [Rieley et al., 2011]. Subjects were recruited from the existing NF1 patient population at Cincinnati Children's Hospital Medical Center (CCHMC) as well as Boston Children's Hospital and University of Utah. Inclusion criteria were i) a clinical diagnosis of NF1 based on NIH guidelines; and ii) confirmation of monozygosity by analysis of short tandem repeats at 16 chromosomal loci. Study participation was offered to individuals via phone or in person. Research protocol and all study materials were approved by both the CCHMC Institutional Review Board and the United States Department of Defense Human Research Protection Office, and informed consent was obtained.
Samples
Blood samples were obtained from each individual and cultured for future chromosome analysis, and genomic DNA was isolated according to standard protocols. In addition, buccal swabs or saliva samples were obtained from each twin to assess for intra-individual mosaicism between blood and oral mucosa.
Genome-Wide SNP Data
All samples were genotyped using the Illumina Human 610K-Quad Bead SNP Chip, and only autosomal SNPs (chromosomes 1–22) were used. The HLA region on chromosome 6 was excluded from the analysis due to the high rate of population polymorphism within this region, as were sex chromosomes. Samples with a call rate of <0.997 were discarded. Data were analyzed using Illumina's Genome Studio software. This platform is able to detect changes of >80 kb, with gaps in coverage of 1 megabase or less. All SNP probes are replicated 5–20 times throughout the chip. This data set corresponds to Build 36 of the Human Genome Project. Copy number changes were identified using the cnvPartition Algorithm v.2.3.4.
All CNVs were visually inspected with regard to log R ratio and B allele frequency, and compared to co-twins, parents and saliva samples when available by a single reviewer (ERS) to confirm familial or de novo nature of each CNV. The Database of Genomic Variants (http://projects.tcag.ca/variation/) was utilized to categorize CNVs as novel or benign polymorphism with regard to previously reported losses, gains, inversions, and segmental duplications. The cnvPartition algorithm also generated a confidence score for each CNV. CNVs with a confidence score <100 (non-conservative) were excluded from the final analysis due to the increased chance of artifact in this group. Known and predicted genes within conservative CNV regions were catalogued for further analysis.
DNA samples from twin pairs were additionally sent to the University of Alabama at Birmingham Medical Genomics Laboratory for genomic DNA-based NF1 sequence analysis. All exons of the NF1 gene were amplified by PCR, followed by high resolution melting curve analysis using LightScanner® technology, followed by bidirectional sequencing of all samples displaying an aberrant melting curve.
RESULTS
SNP array analysis was performed on blood and saliva or buccal samples from 22 enrolled MZ twins and on blood samples from 15 of their parents. Microarray was successfully performed from blood samples of all participants; however, buccal samples yielded DNA of insufficient quality for microarray, so saliva was collected for subsequent subjects.
Phenotypic and Genotypic Information
Clinical features of each twin pair are summarized in Table I. Overall, 9 of 11 pairs were discordant for two or more features. Pair E, the youngest at age 5 years, was concordant for all parameters studied (Table I). Number of café-au-lait macules, number of cutaneous neurofibromas and presence or absence of Lisch nodules were highly concordant across the twin pairs, in agreement with previous reports [Easton et al., 1993]. NF1 genotyping was performed on 10 twin pairs, with results listed in Table I; none had the NF1 full gene deletion associated with more severe phenotype. No twin pairs were discordant for NF1 mutation. In pair C, no NF1 mutation was detected by sequence analysis, despite clearly meeting NF1 diagnostic criteria.
| Twin pair | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 19 | 10 | 9 | 7 | 5 | 19 | 8 | 15 | 26 | 14 | 6 |
| Race | W | W | W | W | AA | W | W | W | W | W | W |
| Family history | Paternal | Maternal | Sporadic | Sporadic | Maternal | Unknown | Sporadic | Paternal | Sporadic | Sporadic | Paternal |
| Café-au-lait spots # | 15+/15+ | 15+/15+ | 15+/15+ | 15+/9 | 9/10 | 15+/15+ | 15+/10 | 8/10 | 7/5 | 15+/15+ | 15+/15+ |
| Lisch nodules | +/+ | −/− | +/+ | −/− | −/− | +/− | −/− | +/+ | +/+ | −/− | −/+ |
| Cutaneous neurofibroma # | 10+/10+ | −/− | N.A. | −/− | −/− | −/3 | −/− | 3/1 | 200+/200+ | −/− | 3/3 |
| Optic glioma | −/− | −/+ | −/− | −/+ | −/− | −/− | −/− | −/− | −/+ | −/+ | +/− |
| Plexiform neurofibroma # | mult/mult | 1/− | −/− | −/1 | −/− | −/1 | 2/− | −/− | −/2 | −/2 | −/1 |
| MPNST | +/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Pectus deformity | +/+ | −/− | +/+ | −/+ | −/− | −/− | +/+ | −/− | +/− | −/+ | +/+ |
| Scoliosis (degree curve) | −/− | −/− | 21/− | N.A. | N.A. | −/− | 55/− | 6/8 | 30/31 | 17/44 | −/− |
| Intellectual disability | −/− | −/− | −/− | −/− | −/− | +/+ | −/− | −/− | −/− | −/− | −/− |
| Learning disability | +/+ | −/+ | +/+ | −/+ | +/+ | +/+ | N.A. | +/− | −/+ | +/+ | N.A. |
| ADHD | +/+ | +/+ | −/+ | −/N.A. | −/− | +/+ | Possible/+ | −/− | −/− | +/+ | +/− |
| Vascular | +/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Paraspinal tumor | +/+ | −/− | −/− | −/− | N.A./− | −/N.A. | +/− | −/− | −/− | −/+ | −/− |
| Tibial dysplasia | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | N.A. | −/+ |
| Mutation | 289_586del | 288 + 1G > A | None | 7375_7378delCATG | 3457C > G | N.A. | 3198−2 A > G | 4269G > A | 5242C > T | 5242C > T | 4773(−6)_4775 delCCTTAGGTT |
- NF1 traits are shown for each twin pair. Discordant traits are highlighted in gray. Unhighlighted traits are concordant for presence of absence of the trait within the twin pair. Twins A, B, C, D, F, G, I, and J have been previously reported in Rieley et al. [2011]. Abbreviations: +, presence of trait; −, absence of trait; mult, multiple; N.A., not available. W, white or Caucasian; AA, African American.
CNV Type and Distribution
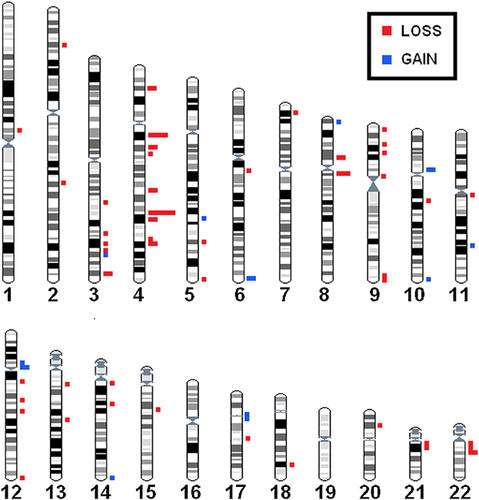
The overall number of CNVs was relatively similar across the twin pairs, with each pair having between 12 and 26 raw CNVs (mean:18.9). All CNVs identified were concordant within the twin pair and inherited from a parent, when parents were available for comparison. Each pair had between 2 and 12 conservative CNVs (mean: 7.4). The majority (61/81 = 75.3%) were copy number losses smaller than 200 kb (Table II). Among conservative CNVs, 76 (93.8%) were identified as benign polymorphisms, occurring in >1% of the general population (Supplemental Table SI). In general, identified CNVs were found to be widely distributed across the genome, with a concentration on 4q (Fig. 1).
| Family | A | B | C | D | E | F | G | H | I | J | K | Totals | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conservative CNVs | 22 | 21 | 17 | 12 | 26 | 16 | 18 | 16 | 21 | 24 | 15 | 208 | 18.9 |
| Copy number losses | 11 | 8 | 9 | 3 | 12 | 2 | 7 | 6 | 9 | 10 | 4 | 81 | 7.4 |
| Polymorphisms | 9 | 8 | 9 | 2 | 9 | 2 | 6 | 4 | 6 | 8 | 3 | 66 | 6.0 |
| CNV incl. genes | 6 | 3 | 3 | 1 | 5 | 1 | 3 | 3 | 5 | 4 | 3 | 37 | 3.4 |
- Conservative CNVs are those with CNV confidence values >100, which are less likely to be artifact. Polymorphisms are changes found in >1% of the general population.
Genes Within CNVs
CNVs were further interrogated for involvement of known or predicted genes. Thirty-seven of 81 conservative CNVs (45.7%) overlap known or predicted sequences (Supplemental Table SII). Of those CNVs, all but three were considered to be benign polymorphisms. The three remaining CNVs occurred at 11q22.3 (pair E), 2q24.1 (pair H), and 3q22.1 (pair B). The genes involved are c11orf87, an uncharacterized protein; CPNE4, a calcium-dependent phospholipid-binding protein; and GALNT5 (GalNAc Transferase 5) a membrane-bound polypeptide N-acetylgalactosaminyltransferase which catalyzes O-glycosylation. The GalNAc family has been implicated in metastasis [Gaziel-Sovran et al., 2011; Matsumoto et al., 2012].
DISCUSSION
The goal of this pilot study was to explore whether CNVs might help explain the variability in NF1 phenotypic heterogeneity. The SNP-based microarray is a novel tool for investigating the variability of NF1 disease manifestation. Using sets of monozygotic twins, we were able to test for differences between and within twin pairs. Between twin pairs, we identified several CNVs which we speculate could be plausible disease modifiers. However, within twin pairs, we did not identify differences, thus we are not able to draw a significant conclusion on the effects of de novo CNVs on phenotypic heterogeneity in NF1.
Two potentially interesting (albeit polymorphic) CNVs were observed in twin pair A. They have severe disease involvement with multiple paraspinal tumors, MPNST in one twin, and mild intellectual disability in both. The first is a large duplication 4.1 MB away from the NF1 gene on chromosome 17 which contains three genes: FAM27L, FLJ36000, and MTRNR2L1. As it was found in the unaffected parent, it was concluded that this CNV occurred in trans to the familial NF1 mutation. It could be theorized that this CNV predisposed the twins to instability near the NF1 Locus, making them more susceptible to loss of heterozygosity (LOH) or epigenetic silencing of NF1, leading to extensive plexiform neurofibroma formation and, ultimately, malignant transformation. Another interesting polymorphism in this family, at 10q11.22, involves several genes: GPRIN2, ANXA8, ANXAL1, ANXAL2, SYT15, and L25628. GRPRIN2 is G protein regulated inducer of neurite outgrowth. ANXA8, -L1 and -L2 represent the annexin family of calcium and phospholipid binding proteins. SYT15 encodes Synaptogamin 15, a membrane trafficking protein expressed in neurons. While it is interesting to speculate on the possible effects of these CNVs on the clinical findings in this family, phenotypes like learning disability and tumorigenesis are complex and likely multifactorial. The clinical significance, if any, of these CNVs is also not well characterized, thus we are not able to make any definitive associations between these CNVs and phenotype based on this data alone.
In this study, we also sought to explore the rate of de novo CNVs in NF1. Although we have not directly compared copy number variation in our twins to that of the general population, each twin represents an ideal control for their co-twin. We originally hypothesized that NF1 haploinsufficiency might predispose patients to genomic instability and a higher rate of de novo CNV occurrence. The instability would manifest as de novo CNVs that arose somatically, after division of the two embryos, or even later in development after separation of the germ layers. If this were the case, we would expect to have detected CNV differences between co-twins or between tissue types in one individual. As we have not yet identified any CNVs as discordant between twins or tissue types, our current data, although limited by small sample size and young patients, does not support this hypothesis.
Finally, we compared our conservative CNVs to those reported in NF1-related tumors in the literature. Microarray analysis has been utilized previously to investigate somatic changes in DNA extracted from these tumors, and certain CNVs can be found repeatedly in malignant peripheral nerve sheath tumors (MPNST) [Mantripragada et al., 2009]. Common areas of copy number change have been identified on chromosomes 17, 19, and 22q [Koga et al., 2002]; more specifically gains are seen in 7, 8q, 15q, 16p, and 17q, and losses in 9p, 11q, 17p, and 10q [Schmidt et al., 2000; Brekke et al., 2010]. These chromosomal locations were not well represented among our conservative CNVs, and of those that did overlap, the CNVs were not matched for either breakpoints or copy number value (data not shown).
Limitations of this study include the small sample size, young age of participants, the fact that not all parents were available for enrollment, and the relatively high degree of phenotypic concordance among these twin pairs. Based on the literature, we can expect at least one de novo CNV event in every 100 people CNV [Marshall et al., 2008; Redon et al., 2011]; this study only assessed 22 individuals. Due to the statistical rarity of identical twins with NF1, a sample size of 100 or more would be difficult to attain, even with the cooperation of multiple centers. Future studies of this population may endeavor to include affected members of the extended families, to allow for use of epidemiological methods and to increase statistical power. Another complicating factor in this analysis is the young age of our study subjects, between five and 26 years, as CNVs are known to accumulate with age [Fraga et al., 2005]. Additionally, young children are more likely to be concordant for the absence of certain clinical features (such as cutaneous neurofibromas and MPNST), and phenotypic discordance would be expected to arise with age. Discordance in traits such as learning disabilities would be hard to interpret in a twin population, given the increased risk for prematurity and birth asphyxia. In some families, the lack of one or both parental samples may have obscured the presence of a de novo CNV, as CNVs of unknown origin were by default attributed to the missing parent(s). Finally, our study was limited by the lower density of SNPs on the array platform used. Changes smaller than 1 kb would have been missed by this technology. Use of more advanced technologies, including whole genome sequencing, may reveal more subtle genetic differences between identical twins in the future.
In this small, exploratory study, we were unable to find differences in CNVs either between twins or between tissue types in a single individual to explain discordant NF1 features. While this study did not provide decisive evidence for somatic genetic alterations as a mechanism for phenotypic variability in NF1, it highlights the need for further genome-wide research into the identification of modifying factors in NF1. While not de novo, some germline and even common population CNVs may still predispose individuals to various phenotypes. The ability to better predict phenotypic manifestations of NF1, specifically increased risk for benign or malignant tumor burden, could drastically improve medical management and family counseling. Furthermore, the elucidation of modifier genes would provide new targets for therapeutic interventions in NF1, and may even be extrapolated to the non-NF population.
ACKNOWLEDGMENTS
The authors would like to acknowledge Sara Manning for her support in enrolling participants, and the CCHMC Microarray Core for their assistance with data analysis and interpretation.
This research was funded by the following sources: United States Department of Defense Award W81XWH-10-1-0867; and the Cincinnati Center for Neurofibromatosis Research (Director, Dr. Nancy Ratner).