Is cutis verticis Gyrata-Intellectual Disability syndrome an underdiagnosed condition? A case report and review of 62 cases
Abstract
Cutis Verticis Gyrata-Intellectual Disability (CVG-ID) syndrome is a rare neurocutaneous syndrome characterized by intellectual disability and scalp folds and furrows that are typically absent at birth and are first noticed after puberty. First reported in 1893, the syndrome was mainly identified in subjects living in psychiatric institutions, where it was found to have a prevalence of up to 11.4%. Most patients were reported in the literature during the first half of the 20th century. CVG-ID is now a less reported and possibly under-recognized syndrome. Here, we report a patient with CVG-ID that was diagnosed using the novel approach of magnetic resonance imaging and we conduct a systematic review of all patients reported in the last 60 years, discussing the core clinical features of this syndrome. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Cutis Verticis Gyrata (CVG) is an abnormality of the scalp characterized by the formation of skin folds and furrows, that cannot be corrected by pressure or traction on the scalp. This abnormality was first described in 1837 by Alibert and has been reported since both as an isolated form or in association with other conditions.
The association of CVG with intellectual disability (ID) was first reported by Mc Dowell and Cowan in 1893, in a patient affected by severe ID and epilepsy with muscle wasting and contractures [Berg and Windrath-Scott, 1962]. Since, that patient report several authors have reported similar instances in isolated patients [Paravicini, 1902; Besta, 1904; Bravetta, 1910; Fisher, 1922]. All published patients with CVG were studied in 1953 by Poland and Butterworth, who classified CVG into primary and secondary forms. In the first, CVG occurred as a consequence of inflammatory or neoplastic processes that produce pathological changes in the scalp structure, while in the latter (30% of all patients) the histopathology of the skin was essentially normal and the patients manifested evidence of ID, schizophrenia or epilepsy in the absence of other disorders [Polan and Butterworth, 1953]. The term CVG-ID was then used to describe the syndrome characterized by primary CVG and ID. For a list of all secondary causes of CVG please see Table I.
| Local |
| Inflammatory: eczema, psoriasis, folliculitis, impetigo, erisipela, penphigus |
| Tumors, including cerebriform intradermal naevus |
| Post-traumatic |
| Fetal brain distruction sequence |
| Systemic |
| Acromegaly |
| Neurofibromatosis |
| Tuberous sclerosis |
| Apert syndrome |
| Pachydermoperiostosis |
| Amyloidosis |
| Ehlers-Danlos syndrome |
| Leukaemia |
| Lymphoma |
| Fallopian tube carcinoma |
| Myxedema |
| Acanthosis nigricans |
| Syphilis |
| Thyroid aplasia |
| Hyper IgE syndrome |
| HIV lipodystrophy |
| Fetal Zika embryopathy |
| Klinefelter syndrome |
| Noonan syndrome |
| Cohen syndrome |
In 1964, Akesson conducted a clinic-epidemiological study of CVG-ID in psychiatric institutions in Sweden. He found a higher incidence of seizures, eye defects, and cerebral palsy among CVG-ID patients; he also noted that in those patients CVG was never reported before puberty. A higher prevalence of CVG was found among institutionalized patients (0.71–3.4%), compared to the prevalence of CVG in the general population (1/100,000 for males and 2.6/100,00 for females) [Akesson, 1964]. These data were confirmed by a subsequent study in Italy, where CVG was found in up to 11.4% of institutionalized patients with ID [Schepis et al., 1990]. This was the last cohort study conducted on CVG-ID and only a few and sporadic patients of CVG-ID have been reported since.
Although the etiology and pathogenesis of CVG-ID is unknown, there is evidence for both endocrinologic dysfunction and genetic etiology. Akesson concluded that the fault underlying CVG-ID might be an endocrine disorder, based on its much greater prevalence among males, rare onset before puberty, and frequent concomitance of the CVG with other endocrine disorders, such as acromegaly. Endocrine dysfunction in CVG-ID was extensively studied but never confirmed [Palo et al., 1970]. Genetic factors are thought to play a role in the etiology of CVG-ID, given its male preponderance and the reports of some familial patients. Further, CVG can be found in a variety of genetic conditions. Pachydermoperiostosis, an autosomal dominant condition characterized by digital clubbing, pachydermia, and periostosis is the most common genetic disorder associated with CVG. CVG has also been observed in Turner syndrome and Noonan syndrome [Parolin Marinoni et al., 1999; Fox et al., 2005]. Although these syndromes are genetically distinct, lymphedema is common to both and has been postulated as the underlying cause of CVG in these patients. The association of CVG-ID with chromosomal fragile sites was reported in 9 out of 21 patients with CVG-ID [Schepis et al., 1989], but it was never confirmed in subsequent studies [Dahir et al., 1992].
Therefore, the etiology of CVG-ID remains unknown, making CVG-ID a forgotten and poorly understood condition.
We report a patient with CVG-ID, who presented with severe psychomotor delay at 2 years of age. CVG-ID was diagnosed at the age of 14, when a brain MRI incidentally revealed the presence of CVG over his scalp. The patient herein reported prompted us to review the CVG-ID syndrome.
With the present report and review of the literature, we further delineate the clinical features of this rare syndrome and highlight the importance of using brain imaging among the intellectually disabled children to diagnose CVG-ID at an early stage.
With this study, we would like to bring CVG-ID back to the attention of medical staff involved with intellectually disabled children and young adults, as a possible cause to list among the differential diagnoses of ID.
METHODS
We searched the NCBI's PubMed database using combinations of the keywords “cutis verticis gyrata” “ribbed or corrugated skin”, “bulldog scalp”, “cutis sulcata”, published up until November 2015. The reference section of each relevant study was examined to identify any further studies meeting the inclusion criteria. For each study identified, only the patients presenting with CVG associated with intellectual disability/psychiatric disorders in the absence of a secondary cause were included. For example, we only included 5 patients out of 10 from the study from Ivanainen [Ivanainen and Arho, 1976] because secondary causes were described for the other patients including encephalitis, birth injury, and tuberous sclerosis. We only included patients presenting with CVG-ID without characteristics of other known entities such as Noonan syndrome. All clinical data collected is summarized in Table II.
| Present case | Kratter [1958a] | Kratter [1958b] | Kratter [1958a] | Berg [1962] | Akesson [1965] | Thomson [1969] | Palo [1970] | Iivanainen [1976] | Schepis [1990] | Dahir [1992] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 1 M | 1 M | 1 M | 1 M | 1 M | 1 M | 1 M | 12 M, 2 F | 5 M | 21 M, 1 F | 1 M |
| Ethnicity | White | White | White | White | NA | NA | NA | NA | NA | White | White |
| Age at clinical evaluation | 4–13 | 26 | 23 | 21 | 43 | 32 | 24 | 18–52 | 30–36 | 27–67 | 27 |
| Family history (ID, CVG, epilepsy) | − | − | − | − | − | Severe ID | − | − | NA | NA | − |
| Genetic analysis | − | NA | NA | NA | Normal karyotype | NA | Normal karyotype | NA | NA | 9/21 with chromosomal fragile sites | Normal karyotype |
| CVG (age of onset) | Yes (<13) | Yes (NA) | Yes (NA) | Yes (NA) | Yes (18) | Yes (NA) | Yes (NA) | Yes (16–28) | Yes (NA) | Yes (NA) | Yes (after puberty) |
| ID (IQ) | ++ (29) | ++ (6) | ++ (5) | ++ (5) | ++ (NA) | ++ (10–20) | ++ (NA) | ++ (< 20) | ++ (<20) | 17/22 ++ (NA) | ++ (NA) |
| + | NA | NA | NA | + | + | + | + | NA | 17 + (5 NA) | + | |
| Microcephaly | + | + | + | + | + | – | + | – | + (1/5) | NA | + |
| Seizures | +# | NA | NA | NA | + | NA | + | + (7/14) | + (1/5) | + (8/22) | − |
| Abnormal brain imaginga | + | NA | NA | NA | − | + | – | + | NA | − | NA |
| Psychiatric disorders | − | NA | NA | + | − | NA | NA | NA | NA | + (5/22) | NA |
| Spastic paralysis | − | + | NA | + | + | NA | + | + (8/14)b | + (3/5) | + (3/22) | + |
| Ophthalmic anomalies | + | NA | NA | NA | − | NA | − | + (5/14) | + (2/5) | + (4/22) | NA |
| Short stature | Tall | NA | + | NA | NA | NA | NA | NA | NA | NA | + |
| Scoliosis/kyphosis | + | NA | NA | NA | + | NA | − | NA | + (1/5) | NA | NA |
| Additional features | VSD | Thyroid aplasia and heart failure |
| Schepis [1995] | Striano 1996 | Chang [1996] | Saraswat [1996] | Farah [1998] | Filosto [2001] | Keller [2001] | Vanhoenacker [2007] | Larsen [2007] | López [2011] | TOTAL | ||
| Sex | 3 M | 1 M | 1 M | 1 M | 1 M | 1 M | 1 M | 1 M | 1 F | 1 F | 1 M | 57 M; 5 F |
| Ethnicity | White | White | Mexican-American | Black | NA | NA | NA | NA | NA | Maori | NA | Varies |
| Age at clinical evaluation | 62–65 | 28 | 20 | 22 | 28 | 27 | 30 | 35 | 54 | 11 | 16 | Range:11–16y Mean:35.5 |
| Family history (ID, CVG, epilepsy) | NA | − | NA | NA | − | − | − | − | NA | − | − | − |
| Genetic analysis | Normal fraX | Normal karyotype | Normal karyotype | Normal karyotype | NA | Normal karyotype | Normal karyotype | 49 XXXY | NA | NA | Normal karyotype | |
| CVG (age of onset) | Yes (NA) | Yes (after puberty) | Yes (15) | Yes | Yes (NA) | Yes (5) | Yes (14) | Yes (NA) | Yes (NA) | Yes (11) | Yes (6) | Range: 5–12 Mean:19y |
| ID (IQ) | 2/3 ++ (NA) | ++ (NA) | ++ (NA) | + (NA) | ++ (35) | ++ (NA) | ++ (NA) | ++ (NA) | + (NA) | + (NA) | + (NA) | 100% (15) |
| Microcephaly | + (1/3) | − | − | − | NA | + | − | − | NA | NA | NA | 28% |
| Seizures | NA | +# | + | + | + | + # | + # | + | NA | + | NA | 44% |
| Abnormal brain imaginga | NA | + | + | + | NA | + | + | − | + | NA | − | 46% |
| Psychiatric disorders | + (1/3) | NA | − | NA | − | NA | − | NA | − | − | − | 11% |
| Spastic paralysis | NA | NA | + | − | NA | + | − | NA | NA | NA | − | 40% |
| Ophthalmic anomalies | + (2/3) | NA | − | − | − | + | − | + | − | + | − | 32% |
| Short stature | NA | + | NA | NA | NA | + | Tall | Tall | Normal | Normal | Normal | Varies |
| Scoliosis/kyphosis | NA | NA | NA | NA | NA | NA | + | NA | NA | + | − | |
| Additional features | Long limbs | ASD, VSD |
- a (Including skull X rays, pneumoencephalography and MRI, calcifications, cortical-subcortical atrophy, polymicrogyria).
- b Described with cerebral palsy.
- + # Drug resistant epilepsy.
PATIENT REPORT
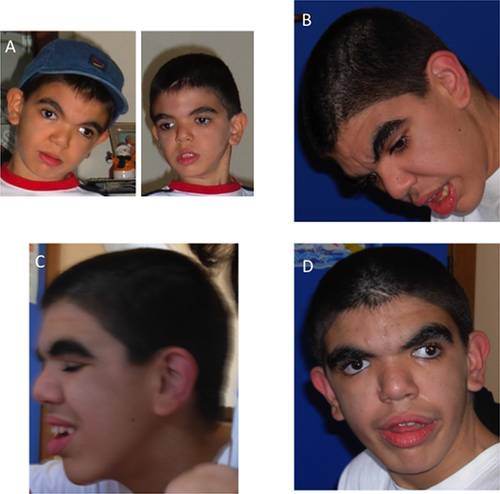
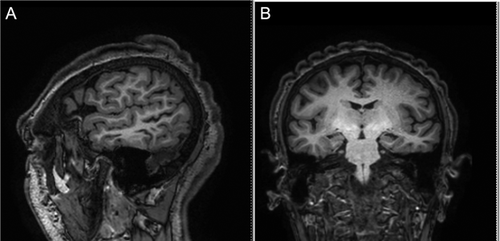
A 14-year-old boy was first seen at the age of two in the Pediatric Genetics outpatient clinic because of severe psychomotor delay and epilepsy. He was the only son of healthy, non-consanguineous parents. There was no family history of psychomotor delay, epilepsy or CVG. An unremarkable antenatal history was reported. He was born at 37 weeks of gestation by C-section because of lack of head engagement into the pelvic cavity. His birth weight was 4,690 g (97th centile), height was 55.7 cm (>97th centile), occipital-frontal circumference (OFC) was 36 cm (75–99th centile), APGAR scores were 5 and 8 at 1st and 5th min, respectively. He was hypotonic and suffered from minimal respiratory distress. An echocardiograph revealed a ventricular septal defect (VSD) and an atrial septal defect (ASD). During the first 2 months of age, he developed gastroesophageal reflux, requiring fundoplication surgery. At the age of 2 years, he developed drug-resistant epilepsy which was treated with little benefit with topiramate and oxcarbazepine. On examination, his growth was normal for height, weight and OFC; he was noted to be dysmorphic with low anterior hairline, bitemporal narrowing, thick and highly arched eyebrows, long eyelashes, hypertelorism, epicanthus inversus, downslanted palpebral fissures, depressed nasal bridge, and low set ears. By the age of seven, he was frequently hospitalized because of recurrent episodes of tonic-clonic seizures causing frequent aspiration pneumonia and chronic pneumopathy. He was also found to have scoliosis convex to the right and hand spasticity with finger swan-neck deformity. His growth parameters were above the 97th centile for height and weight and on the 95–90th centile for OFC. The following tests were carried out: neuropsychological, which showed an IQ of 29 with complete absence of language; echocardiography, which showed VSD and pulmonary hypertension; polysomnography, which showed normal polysomnographic respiratory values and electroencephalographic abnormalities involving the right temporo-occipital region with irregular waves (seldom sharp) at 4–5 c/sec, 30–50 mcV.; brain MRI, showing hippocampal atrophy, cortical ischemic anomalies in the temporal region possibly due to status epilepticus, and a disproportion between the neurocranium and the viscerocranium, which was prominent due to long face, pronounced jaw, and receding forehead. These features, in addition to the small frontal lobes provided a microcephalic appearance of the head; ophthalmologic assessment, showing strabismus and mild myopia; brainstem auditory evoked response which was normal; and carpal bone X-rays which showed a normal bone age. A metabolic screen including mucopolysaccaridosis was performed and found to be normal. A number of genetic analyses were performed and were all normal, including FISH for microdeletion 17p11.2, karyotype, molecular analysis of the NIPBL, RSK2, and of the SGSH genes and array-CGH. Nasal brush for primary ciliary dyskinesia was also normal. At the age of nine, coarsening of the facial features was noted (Fig. 1). At the age of fourteen, a brain MRI was repeated and revealed microcephaly, left temporal lobe atrophy with widening of the cerebral sulci and thinning of the cerebral cortex an hippocampal atrophy with widening of the temporal horn and lateral ventricles. CVG was noted as an incidental finding (Fig. 2). Given the association of CVG with endocrinological alterations, the following measurements were carried out: TSH, FT3, FT4, PRL, GH, IGF2, insulin, glycaemia, and oral glucose tolerance test were all normal. As known secondary causes of CVG were excluded, a diagnosis of CVG-ID was made.
RESULTS
The clinical data collected through the literature search were presented in Table II. A total of 62 patients presenting with CVG-ID not attributable to a secondary or a known cause of CVG were included in our review.
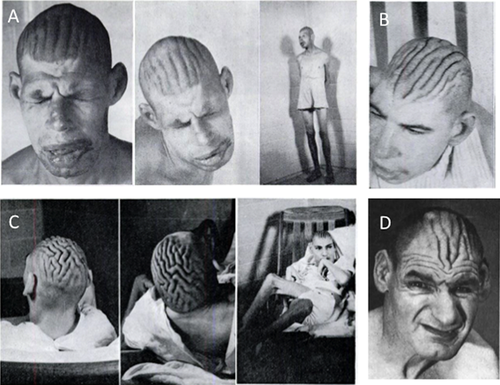
At a gross examination, CVG presents as furrows (3–4 mm deep) separated by folds (6–14 mm wide), typically localized over the vertex in the frontal and parietal regions. Folds are usually symmetrical and arranged in parallel in the antero-posterior direction; they adhere to one another and are incapable of being smoothed out by traction on the scalp [Kratter, 1958a; Berg and Windrath-Scott, 1962]. The histopathological examination shows normal skin, thickened connective tissues, hyperplasia of adnexal structures, increased number of hair follicles, and sebaceous glands in the dermis or epidermal thickening [Polan and Butterworth, 1953]. Occasionally, few small foci of lymphocytes in dilated tissue spaces around the capillaries are observed [Berg and Windrath-Scott, 1962] and some increase in collagen tissue and fragmentation of elastic fibers may also be observed [Akesson, 1965; Thomson and Macgillivray, 1969; Schepis et al., 1990; Diven et al., 1991].
With regards to the facial appearance, most features become more apparent with age. Patients present with coarse and long face, prominent supraorbital ridges, a low, narrow and sloping forehead, high nasal bridge, long nose, broad jaw, large and protruding ears, and thick vermillion of the upper and lower lips (Fig. 3) [Kratter, 1958b; Berg and Windrath-Scott, 1962].
Imaging
Skull X-rays shows normal skull shape and thickness, normal pituitary fossa, and clinoid process [Berg and Windrath-Scott, 1962; Akesson, 1965; Schepis and Siragusa, 1995]. Pneumoencephalography performed in 11 patients revealed narrow posterior cranial fossa in about half of them and a small cerebellum in two individuals.
Brain imaging
Brain anomalies detected by computed tomography (CT) or MRI are found in about 38% of patients. Cortical or subcortical atrophy is the most common finding and has been described in all brain areas including occipital, parietal, and in the posterior fossa structure. Occasional findings include polymicrogyria [Striano et al., 1996], microcephaly, colpocephaly of the occipital horns, and pronounced cerebellar folia with small splenium [Farah et al., 1998] or extensive periventricular calcifications [Vanhoenacker et al., 2007].
Scalp imaging
CT scan shows a typical pattern of marked skin convolutions in the scalp, resulting in a typical gyriform or cerebriform pattern [Chang, 1996; Vanhoenacker et al., 2007]. MRI findings consist of: 1-thickening of the galeal tissue, which raises the overlying adipose and cutaneous tissue in ridges and furrows [Striano et al., 1996], 2-coarse thick scalp rugae [Farah et al., 1998], 3-an abnormal thickening of the skin, and connective tissue [Filosto et al., 2001]. The skull is typically unaffected [Chang, 1996].
DISCUSSION
We report a patient with CVG-ID syndrome and review all cases described in the literature up to 2016, comprising a total of 62 patients. Notably, 80% of patients were reported in the literature before 1990 and they were mostly subjects living in psychiatric institutions [Palo et al., 1970; Schepis et al., 1990; Dahir et al., 1992] or hospitalized in neurology wards [Chang, 1996; Farah et al., 1998; Filosto et al., 2001]. CVG-ID is now a less reported and possibly under-recognized syndrome, in our opinion due to the deinstitutionalisation of severely intellectually disabled people.
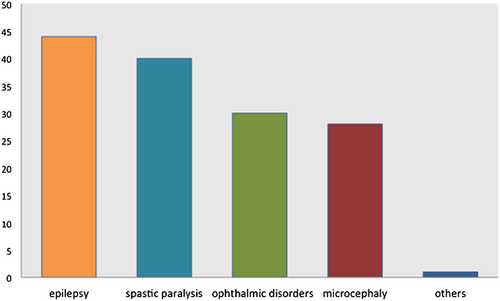
Our data suggest that CVG-ID is a constitutional syndrome affecting predominantly males, whose major clinical features are as follows: (i) severe to profound psychomotor delay (100%); (ii) CVG, which is absent at birth and becomes apparent before or shortly after puberty (100%); (iii) epilepsy, often of early onset, that can be drug-resistant (44%); (iv) Spastic paralysis (40%); (v) eye disorders (30%), most frequently strabismus; (vi) microcephaly (28%) (Fig. 4).
All patients present with severe to profound psychomotor delay, with some patients never learning to walk or talk [Berg and Windrath-Scott, 1962; Akesson, 1965; our patient]. As adults, most patients are unable to speak and to perform activities of daily living. Psychiatric disorders mainly consist of schizophrenia [Kratter, 1958a; Schepis et al., 1990; Schepis and Siragusa, 1995].
CVG is usually firstly observed in patients who have reached or already passed the puberty. It is likely that the infrequent recognition of CVG before puberty results from the insidious nature of this alteration. Our report clearly shows that brain imaging through MRI revealed the skin changes before they were noticed by the parents, allowing us to diagnose CVG-ID syndrome at an early stage. We suggest using brain imaging to detect early scalp changes consistent with CVG in severely intellectually disabled children and young adults to allow for early diagnosis of CVG-ID syndrome.
The seizure types can be varied, presenting as chronic intermittent tonic-clonic seizures [Berg and Windrath-Scott, 1962; Thomson and Macgillivray, 1969; Chang, 1996; Saraswat and Kuldeep, 1996; Keller et al., 2001] or drug-resistant tonic clonic seizures and absences, with neonatal/early childhood onset [Farah et al., 1998; Filosto et al., 2001; our patient; Striano et al., 1996]. One patient has been described with Lennox-Gastaut syndrome [Filosto et al., 2001]. The EEG abnormalities are varied and aspecific accordingly.
Spastic paralysis is often associated with multiple contractures. The vast majority of patients paraplegic; one individual has quadriplegia [Berg and Windrath-Scott, 1962]. Additionally, 13% of patients are described as having cerebral palsy with the degree of motor involvement not further discussed [Palo et al., 1970].
Strabismus/diplopia is the most frequently reported eye anomaly (23%) [our patient; Palo et al., 1970; Iivanainen and Arho, 1976; Schepis et al., 1990], followed by optic nerve atrophy (18%) [Palo et al., 1970; Iivanainen and Arho, 1976; Farah et al., 1998; Larsen and Birchall, 2007], nystagmus (14%) [Iivanainen and Arho, 1976; Schepis et al., 1990; Farah et al., 1998] and cataract (14%) [Palo et al., 1970; Schepis et al., 1990; Schepis and Siragusa, 1995]. Myopia, microphthalmia and pterygium are rarely reported (1% each) [Palo et al., 1970; Iivanainen and Arho, 1976; Schepis and Siragusa, 1995; our patient].
Nearly all instances of CVG-ID reported in the literature present in males. The uneven distribution of CVG-ID between males and females has not yet been explained. Although the skin anomaly may be easier to detect in males in a study conducted by Schepis, where all patients suspected of having CVG underwent total scalp trichotomy, they identified only 1 female and 22 males with CVG-ID [Schepis et al., 1990]. Furthermore, in the adult the anomaly is often so noticeable that the most superficial palpation would bring it to notice. Our review comprises 56 males and 5 females. Notably, one female included in our study is a 54-year-old psychiatric woman also affected by panhypopituitarism [Vanhoenacker et al., 2007] and one is amenorrhoeic at the age of 21 with normal sex-chromatin [Palo et al., 1970], indicating the likelihood of an endocrine dysfunction in those patients.
The genetic basis of CVG-ID remains elusive. Despite the suggestion made by Polan Butterworth in 1953 that CVG-ID is an anomaly of development brought about by the same constitutional fault this has not been identified. It is likely that CVG-ID is a genetically heterogeneous disorder. The large majority of the cases are sporadic occurrences in a family, suggesting de novo mutations in such cases. Further, the overwhelming majority are males, suggesting X-linked inheritance. However, among all patients described in the literature, only two familial occurrences have been reported. Akesson described five males in three sibships of two generations with a combination of CVG, ID, and thyroid aplasia [Akesson, 1965] which is strongly suggestive of the disease being inherited through an X-linked recessive gene. Besta, [1904] described two brothers who had severe ID, CVG, and microcephaly. Although X-linked inheritance could be the mechanism explaining the presence of the two affected brothers in this family, autosomal recessive inheritance or even germline mosaicism for an autosomal gene cannot be excluded. Moreover, the presence additional microcephaly in these brothers, which was not mentioned in the family of Akesson may suggests further genetic heterogeneity, In this regard, we note that two brothers were reported with ID, microcephaly, CVG, retinitis pigmentosa, cataracts, and sensorineural deafness [Mégarbané et al., 2001]. The two brothers were later found to carry homozygous mutations in the Cohen gene VPS13B [Mégarbané et al., 2009]. CVG could be either part of the syndrome or be caused by mutations in a different gene.
Two of the patients presented in this series (our patient and the patient reported by Larsen in 2007) have a heart defect consistent with ASD or VSD. As none of the previously reported patients underwent an echocardiogram, it is impossible to know whether this is a feature of the CVG-ID syndrome or a sporadically associated defect. Regardless, we strongly suggest performing a heart scan and monitoring heart function periodically to any person diagnosed with CVG-ID.
With this study, we want to emphasize CVG-ID syndrome as a possible diagnosis among children with severe psychomotor delay and we suggest using brain MRI (often already performed as a diagnostic tool) to look at scalp changes consistent with CVG.
The approach to the patient with CVG and psychomotor delay should include a family history, physical examination of the affected area, and detailed neurological and cardiac examination (including an echocardiogram). Additionally laboratory, endocrine radiological studies should be performed to exclude secondary causes of CVG. In terms of management and treatment because skin lesions are generally asymptomatic, treatment may be requested solely for aesthetic reasons. Surgical resection of the lesions with stretching of the remaining skin is the treatment of choice [Mutter et al., 1993].
ACKNOWLEDGMENTS
We thank the patient and his parents for their kind cooperation.