Temporomandibular joint ankylosis as part of the clinical spectrum of Carey–Fineman–Ziter syndrome?
Abstract
The Carey–Finema–Ziter syndrome (CFZS, MIM 254940) is an apparently autosomal recessively inherited disorder consisting of the combination of non-progressive congenital myopathy with Moebius and Pierre Robin sequence, facial anomalies and growth delay. Mental development has been described as normal or delayed. Temporomandibular joint (TMJ) ankylosis is the immobility of the joint caused by ankylotic fusion of the mandible to the cranial base or zygoma. It is a serious and disabling condition that may cause problems in mastication, digestion, speech, appearance, and oral hygiene. Most often is a true ankylosis of the TMJ but other pathological mechanisms are described (i.e., the fusion of the coronoid process to temporal bone or with the zygoma, or a variety of soft tissues disorders like Fibrodysplasia Ossificans Progressiva). Here we report a 2-year-old girl fitting with a clinical diagnosis of CFZS associated with a limited mouth opening in which temporomandibular joint ankylosis was suspected. Because it has been postulated that many clinical features in CFZS may only be secondary effects of brainstem anomalies and muscle weakness during development, the limited opening of the mouth observed in our patient could represent a rare clinical feature of CFZS itself. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
The Carey–Fineman–Ziter syndrome (CFZS, OMIM 254940) was first described by Carey et al. [1982]. It is characterized by hypotonia, Moebius sequence (bilateral congenital facial palsy with impairment of ocular abduction), Pierre Robin complex (micrognathia, glossoptosis, high-arched, or cleft palate), delayed motor milestones and failure to thrive. [Carey et al., 1982; Schimke et al., 1993; Carey, 2004]. Affected patients can also show microcephaly, or macrocephaly, and facial dysmorphisms including downslanting palpebral fissures, ptosis of the eyelids, epicanthal folds, upturned nose, low-set posteriorly rotated ears and lack of expression; variable brain anomalies have been described (ventriculomegaly, reduced white matter, neuronal heterotopias, small foci of necrosis with microcalcifications, small pons and brainstem with enlarged pre-pontine and pontocerebellar cisterns) as well as absence of the pectoralis major muscle with an ulnar deviation of the hand (Poland sequence) [Baraitser and Reardon, 1994; Maheshwari et al., 2004]. Occasionally laryngostenosis and clubfeet are reported. Hypotonia, Pierre Robin complex (with or without cleft palate) resulting in feeding and swallowing problems, ophtalmoplegia and facial weakness were present in the great majority of patients. Intelligence may be normal but mental disability has been reported [Dufke et al., 2004]. Exact data about prevalence and incidence are not known: up to now, less then 20 cases have been reported in the literature.
CFZS is thought to be an autosomal recessively inherited disorder also if the biologic basis are still unknown. Diagnosis can be made only at clinical level; magnetic resonance imaging (MRI) could be necessary to detect the brain anomalies.
It has been postulated that many clinical features in CFZS may only be secondary effects of muscle weakness during development [Carey, 2004; Verloes et al., 2004]. The facial appearance with downslanting palpebral fissures, ptosis and lack of expression may be the result of facial muscle weakness and immobility (amimic face). Facial weakness also may give rise to altered mandibular movement consequent mandibular hypoplasia and Pierre Robin sequence. Similarly, clubfeet and other joint contractures are seen in other non-specific myopathies of prenatal onset, associated with paucity of fetal movements. Hypotonia in CFZS has been attributed to a congenital non-progressive myopathy. Muscle biopsies performed in four of the seven cases above reported showed a pattern suggestive for generalized non-specific myopathy (such as type I fiber atrophy, a finding commonly associated with congenital myopathies). It has been hypothesized that CFZ syndrome, instead of being a distinct nosologic entity, may be the severe end of the spectrum in Moebius syndrome [Verloes et al., 2004].
Temporomandibular joint disorders is a broad diagnosis that includes functional disturbances and anatomical disorders of the temporomandibular joint. Anatomical causes of temporomandibular joint disorders result in partially or totally restricted motion and include deformation of the joint itself and pathological involvement of the bone or soft tissue. Functional disturbance are characterized by restricted active motion and usually secondary to pain resulting from malposition of the disk, capsulitis or muscular diseases. Pediatric TMJ dysfunction results from either soft tissue or skeletal disorders, and can be congenital or acquired [Allori et al., 2010; Stoll et al., 2012].
A well-known association between TMJ ankylosis and clinical syndrome in children is the association between TMJ ankylosis and Fyblodysplasia Ossificans Progressiva (FOP), a rare inherited disorder of the connective tissue, with autosomal dominant inheritance, in which skeletal abnormalities are associated with progressive ossification of striated muscle and soft connective tissue [Braga et al., 2011; Pignolo et al., 2013].
The association between CFZS and TMJ disorders has never been reported before: this is the first case described in which TMJ ankylosis is a feature of this complex syndrome. Moreover clinical exome sequencing analysis performed in our patient did not permit to identify any pathogenetic variant in known causing disease's gene.
CLINICAL REPORT
This girl was the first child of healthy Italian non consanguineous parents, born at 38 weeks gestation. Family history is unremarkable. Pregnancy was complicated by risk of miscarriage at 9 weeks gestation and the evidence of clubfeet at the 4th month. She was delivered by emergency caesarean section for fetal distress during labor; at birth there was evidence of fluid stained with meconium. Apgar score was 5 at the 1′ and 8 at the 5′. Birth weight was 2,300 g (50° centile) length was 44 cm (44° centile) and head circumference was 32 cm (5° centile). Mechanical ventilation (with difficult intubation) was required soon after birth because of respiratory distress; at 1st day of life she was admitted to NICU where she stayed until the 4th month of age.
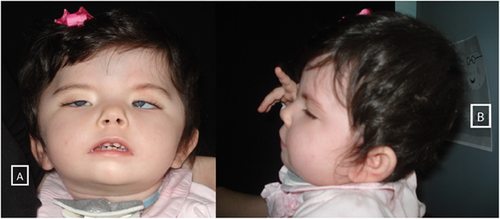
Clinical examination at birth revealed plagiocephaly, facial findings (flat nasal root, bilateral epicanthus, bilateral ptosis) limited mouth opening, Pierre Robin complex (retrognathia, cleft palate, glossoptosis). She had long tapered fingers and bilateral talipes equinovarus.
Neurological examinations revealed severe hypotonia, facial palsy and oculomotor palsy (from III to VII cranial nerve palsy). Swallowing was absent. Cranial MRI revealed multiple anomalies: subependymal heterotopia, thinning of the white matter, bilateral ventricular dilatation. Echocardiography showed a small ASD and left branch pulmonary artery hypoplasia.
During the first weeks in NICU she need continuous respiratory support due to upper airways instability. She underwent tracheo-laryngoscopy which revealed laryngostenosis (two focal tracheal narrowings at the upper third), so tracheostomy was performed when she was 23 days of age to achieve airway stability. A percutaneous endoscopic gastrostomy was made at the age of 2 months in view of persistent difficulties in suck and swallowing associated with a limited mouth opening. She had been fed with infant formula from the birth with poor growth: X-ray study of intestinal transit revealed severe gastroesophageal reflux so a permanent feeding gastrostomy was placed and an appropriated therapy with lansoprazole and domperidone was started. Due to repeated episodes of aspiration pneumonitis, a Nissen fundoplication surgery was required at 14 months.
Several investigation including metabolic screening (urinary organic acids, plasma and urinary aminoacids) and genetic analyses were performed without conclusive results. Peripheral blood karyotype was normal (46, XX), FISH analyses for del(22q11) and molecular analyses of gene MLL2 (associated with Kabuki Syndrome) were negative. Comparative genomic hybridization was also negative (resolution 180 k).
We saw the patient at the age of 13 months. Based on the combination of key features such as facial palsy, cardiopathy, cleft palate, subependymal heterotopia, laryngostenosis and failure to thrive a clinical diagnosis of Carey–Finema–Ziter syndrome was made. Next generation sequencing study with a whole exome approach was carried out on parents-child trio by using Agilent's SureSelect Clinical Research Exome kit (Agilent Technologies, Santa Clara, CA). Sequencing was performed on an Illumina NextSeq500 platform (Illumina Inc., San Diego, CA). The achieved coverage was >10× for more than 98% of the targeted exons and exon-intron boundaries. The analysis focused on known disease-genes, using a recessive and dominant de novo model. No potentially causative variants were identified by this approach.
At our last evaluation, at the age of 21 months, she was 11.5 kg (50° centile). She was in good general conditions, no more hospital admissions since the fundoplication surgery. She showed facial anomalies including flat nasal root, bilateral epicanthus, Pierre Robin complex (Fig. 1). There were bilateral facial palsy with minimal facial expression and bilateral ptosis. She was able to move all four limbs but she couldn't keep sitting position due to severe axial hypotonia; head control was poor as well. Sucking and swallowing were completely absent and there was a limited mouth opening. Language was absent. Tracheostomy and gastrostomy were tidy.
In order to resolve the limited mouth opening, such a disabling affliction causing to our patient problems with mastication, digestion, speech and oral hygiene, we consulted our maxillofacial surgeons.
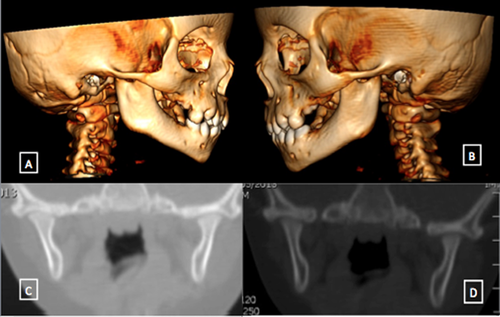
The facial appearance and morphology, dimension and position of the mandible appeared to be normal. However, on the dynamic side, no proper mandibular movements could be appreciated. There was a reduced mouth opening, and such condition persisted even while trying to open the patient's mouth while she was awake. These clinical findings were suggestive for fibrous temporomandibular joint ankylosis. So we decided to perform a TC scan under anesthesia, and eventually a forced opening of the jaws. Coronal TC scan revealed absence of any fibrous or bony temporomandibular joint ankylosis, but joint cavity was decreased especially on the right side, where in some points the head of the condyle was in very close contact the roof mandibular fossa, although without clear-cut fusion points.
Morphologically mandibular fossa had a normal conformation, such as condylar neck had, while condylar head appeared flattened, with a dysomogeneous and inadequate cortical development. CT scan sequences did not show any interference between coronoid and bone wall of zygomatic arch or other bony interferences. Figure 2 shows a 3D vision of the skull and detailed analysis of temporomandibular joints of the patient. Therefore, a forced opening of the jaws was performed, and that maneuver allowed to reach maximal interincisal opening of 28–30 mm, although the movement was not fluid and there was no distinction between rotational and translatory phases.
Clinical, radiological and surgical evaluations excluded the presence of specific articular diseases and underlined the possibility to perform the movement of opening/closing the mouth with a good range of motion. However, characteristics of the movement and radiological findings suggested an abnormal development of temporomandibular articular structures, with hypoplasia of joint capsule and ligaments likely related to the muscular weakness and neurological dysfunction inherent the CFZ syndrome. As a result of these observations we excluded any surgical treatment and opted for physiotherapy target to the jaw, both manual and with “oral whirligig.” Physiotherapy was required in order avoid the progressive degeneration of articular structures and underused muscles, and to maintain an adequate range of motion. Due to the early age of the patients, according to our maxillofacial surgeons, we decided to revalue the girl once the muscular and bone growth will be completed. The cleft palate would have been resolved once it will be resolved ankylosis.
DISCUSSION
To the best of our knowledge this is the first case described in which CFZS is complicated by TMJ ankylosis. The CFZS is a consistent and recognizable phenotype with hypotonia, Moebius sequence, facial anomalies, Pierre Robin complex and delayed motor milestones. Our girl presented with different key features such as facial palsy, cardiopathy, cleft palate, subependymal heterotopia, laryngostenosis, and failure to thrive. Several genetic tests performed, including arrayCGH, gave normal results. For all these reasons we consider as possible the clinical diagnosis of CFZS. We also performed a deep molecular sequencing analysis focused on known disease-genes which covers more than 98% of the targeted exons and exon-intron boundaries using Agilent's SureSelect Clinical Research Exome kit. No potentially causative variants were identified also by this approach excluding that our patient affected with CFZS could be caused by mutation in an already known gene.
Temporomandibular joint ankylosis, which refers to immobility secondary to ankylotic fusion of the mandible to the cranial base or zygoma, is a disabling condition itself [Lima et al., 2011; Hegde et al., 2015]. If associated with CFZS, temporomandibular joint ankylosis worses hypotonia and facial palsy in limiting the mouth opening. Inability to open the mouth adds further difficulties in performing daily life activities for this child.
In our patient the TM ankylosis seems to be congenital. There was no reported exposure to trauma (or surgery) or infections, which are the most frequent causes of TMJ ankylosis in children.
In fact radiological investigations performed to study in deep the limited mouth opening showed absence of a real temporomandibular joint ankylosis. Maxillofacial surgical evaluation revealed an altered development of temporomandibular articular structures, with hypoplasia of joint capsule and ligaments, likely related to the muscular weakness and neurological dysfunction inherent the CFZ syndrome. The absence of a concrete ankylosis made the surgical treatment not indicated in our patient, while physiotherapy target to the jaw and muscular weakness appeared to be the best option.
In 1982 Carey et al. postulated that many clinical features in CFZ syndrome may only be secondary effects of muscle weakness during development. These patients are described to have a congenital non-progressive and non-specific myopathy, leading to muscular weakness. The facial appearance may be the result of facial muscles weakness and immobility (amimic face) and facial weakness itself may give rise to altered mandibular movement with mandibular hypoplasia and Pierre Robin sequence. Similarly, clubfeet and other joint contractures are seen in other non-specific myopathies of prenatal onset, associated with paucity of fetal movements. It has been hypothesized also that CFZ syndrome, instead of being a distinct nosologic entity, may be the severe end of the spectrum in Moebius syndrome: a prenatal ischemic insult to the developing brainstem may explain the association of Moebius sequence and myopathy, correlation with Pierre Robin sequence could be explained by lesions of the lower cranial nerves (and thus not surprisingly associated with Moebius sequence) [Verloes et al., 2004].
Similarly, temporomandibular joint disorders could be expression of muscular weakness [Ryan et al., 1999]: the facial muscles immobility could lead to altered joint development and altered mandibular movements, such as other congenital joint contractures are seen in myopathies with poor fetal movements. Following this hypothesis, the temporomandibular joint disorder and limited mouth opening could represent rare clinical features of CFZS.
ACKNOWLEDGMENTS
We thank Mariani Foundation, Milan for supporting the clinical activity of the Pediatric Genetic Unit of Pediatric Department, MBBM Foundation.