Altered cerebrospinal fluid proteins in Smith–Lemli–Opitz syndrome patients
Abstract
Smith–Lemli–Opitz syndrome (SLOS) is an autosomal recessive, multiple malformation syndrome with neurocognitive impairment. SLOS arises from mutations in the 7-dehydrocholesterol reductase gene which results in impaired enzymatic conversion of 7-dehydrocholesterol to cholesterol. In the current work, we sought to measure proteins that were altered in the cerebrospinal fluid from SLOS patients compared to pediatric controls. Using a multi-analyte antibody-based assay, we found that 12 proteins are altered in SLOS patients. Validation studies were carried out and the findings from this study suggest alterations in extracellular matrix remodeling and further evidence of oxidative stress within the disease pathophysiology. The results of this study will be used to explore biological pathways altered in SLOS and identifies a set of CSF proteins that can be evaluated as biomarkers in future therapeutic trials. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Smith–Lemli–Opitz syndrome (SLOS) is an autosomal recessive, multiple anomalies syndrome with cognitive impairment caused by an inborn error in cholesterol synthesis which was first described by Smith, Lemli, and Opitz in 1964 [Smith et al., 1964]. SLOS results from mutations in the 7-dehydrocholesterol reductase (DHCR7) gene, which encodes the enzyme that catalyzes the reduction of 7-dehydrocholesterol (7-DHC) to cholesterol [Fitzky et al., 1998; Wassif et al., 1998; Waterham et al., 1998]. A recent report by Cross et al. [2015] indicates the carrier frequency of pathogenic alleles in the general population to be on the order of 1% and predicts a disease incidence on the order of one in 40,000 which is consistent with prior clinical studies [Lowry and Yong, 1980; Nowaczyk et al., 2006]. The SLOS phenotypic spectrum is broad [Opitz et al., 2002]. Clinical signs and symptoms range from mild behavioral and learning deficits in combination with minor physical anomalies to a lethal disorder with multiple major congenital anomalies. Cutaneous syndactyly of the second and third toes is the most common reported malformation [Kelley and Hennekam, 2000]. Individuals with SLOS frequently have an atypical facial appearance that includes ptosis, a small upturned nose, and micrognathia [Nowaczyk et al., 2012]. Biochemically, SLOS is characterized by elevated serum levels of 7-DHC and the isomer, 8-dehydrocholesterol (8-DHC), as well as low to normal serum cholesterol levels [Porter, 2008].
The pathological mechanisms connecting the sterol abnormality to the phenotypic manifestations of SLOS have not been fully elucidated, likely in part due to the many biological functions of cholesterol. Cholesterol is an integral component of lipid rafts from which signal transduction pathways are initiated and is also a precursor of bile acids, oxysterols, and steroid hormones [Porter, 2008]. Besides the impact of cholesterol deficiency in SLOS, elevated levels of 7-DHC and 7-DHC oxidation products appear to have toxic effects [Gaoua et al., 1999; Wassif et al., 2003; Korade et al., 2010; Fliesler, 2013]. A recent antioxidant diet study utilizing a mixture with Vitamin E as the active component normalized oxysterol levels in SLOS patient fibroblast cultures and mouse neuronal cultures [Fliesler, 2013; Korade et al., 2014]. Collectively, the data indicate that a combination of decreased cholesterol levels, decreased sterol levels, and increased levels of 7-DHC and its oxidized derivatives collectively contribute to the pathology (i.e., manifestations) of SLOS.
Anecdotal reports suggest some therapeutic benefit of dietary cholesterol supplementation [Svoboda et al., 2012], but a short-term, placebo-controlled trial was not able to demonstrate beneficial effects on behavior [Tierney et al., 2010]. Cholesterol does not cross the blood-brain barrier, thus limiting the potential of dietary cholesterol supplementation to treat the cognitive and behavioral abnormalities observed in SLOS patients. Although behavioral and cognitive problems arising due to the altered sterol biochemistry might be amenable to treatment, there are no effective therapies to modulate central nervous system sterol composition to date. Due to the limited number of subjects, challenges arise in conducting adequately powered clinical trials for rare disorders such as SLOS. The development of a series of disease-relevant biomarkers could provide insight into the pathology of SLOS and provide a method to evaluate potential efficacy of experimental interventions. In this paper we utilize multi-analyte profiling of cerebrospinal fluid (CSF) to identify a set of proteins that may be useful for monitoring disease status in clinical trials and further our understanding of SLOS pathology.
MATERIALS AND METHODS
Clinical Samples
SLOS subjects included in this study were enrolled in clinical studies (98-CH-0081 or 03-CH-0225) which were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Informed consent was obtained from parents or guardians. A diagnosis of SLOS was established by biochemical and molecular confirmation of the clinical diagnosis. Control CSF was obtained from gender- and age-matched patients who were undergoing CSF collection for another clinical indication. Adult control CSF samples were obtained from BioChemed Services (Winchester, VA). All CSF aliquots were stored at −80°C until use. Severity scores were assigned based on malformation analysis as previously reported [Kelley and Hennekam, 2000]. Two groups of SLOS subject samples were used including an initial group of 20 SLOS subject samples followed by a validation group of 15 SLOS patients. Samples from the same subjects, with different collection times were used as well as some samples unique to each group. The demographics, severity score, and sterol levels for the SLOS subject samples analyzed are listed in Supplemental Table SI.
Cerebrospinal Fluid Analyte Measurements
CSF samples were analyzed by Rules-Based Medicine (RBM), Inc. (Austin, TX) using the Human Discovery Multi-Analyte Profile (MAP) panel. A total of 20 SLOS patient samples and 30 pediatric control CSF samples was evaluated. Statistical analysis of analyte levels was performed as described previously [Cologna et al., 2014]. Briefly, measurements were log10 transformed to achieve a normal distribution. For analytes with less than 40% of the measurements reported as below the limit of detection (LOD), the missing values were substituted as LOD/2 [Ganser and Hewett, 2010]. An unpaired t-test (P < 0.05) was used to compare patient and control CSF samples using GraphPad Prism (version 5). Correlation analysis was carried out with GraphPad Prism using the raw data and Spearman (nonparametric) analysis.
Western Blots
Western blot analysis was carried out using 15 μl of CSF per patient and prepared according to the manufacturer's suggested protocol (Invitrogen, Carlsbad, CA). Proteins were separated on NuPAGE Novex 4–12% Bis-Tris gels. Following separation, proteins were transferred to nitrocellulose membranes using the iBlot Dry Transfer System according to the manufacturer's instructions. Membranes were blocked overnight at 4°C using the WesternBreeze blocking reagents (Invitrogen #WB7050) and then incubated with Rabbit Anti-CTGF antibody (1:4000, Abcam #ab6992) overnight at 4°C. Membranes were washed with WesternBreeze wash buffer and incubated with HRP-linked Goat Anti-Rabbit secondary antibody (1:10000, Sigma–Aldrich, Saint Louis, MO, Item #A6154) for 1 hr at room temperature. Blots were developed using Clarity Western ECL Substrate (Bio-Rad, Hercules, CA, Item #170-5060). Band intensities were quantified (ChemiDoc Image Lab software v. 4.1, Bio-Rad) and normalized to the mean intensity of bands from the control patients.
Gelatin Zymography
For gelatin zymography, 25 μl of CSF or 15 μl of serum (diluted 1:15) was mixed with 2x Laemmli buffer (125 mM Tris-HCl pH 6.8), 20% (v/v) glycerol, 4% (w/v) SDS, 0.005% (w/v) bromophenol blue). Non-reduced, non-thermally denatured samples were separated on 10% precast polyacrylamide gels with gelatin (Bio-Rad, Item: #161-1167) in 1× Tris/SDS/Glycine running buffer for ∼2.5 hr at 120 V on ice. Gels were incubated in renaturing buffer (2.5% Triton X-100) at room temperature for 30 min, then the incubation was repeated. After rinsing five times with water, gels were incubated in renaturation buffer (Tris 50 mM, NaCl 200 mM, CaCl2 5 mM, pH 7.6) for 20 hr at 37°C. Gels were fixed for 1 hr in 30% methanol/7.5% acetic acid and incubated in Coomassie Blue R-250 staining solution (Bio-Rad, Item: #161-0436) for 15 min at room temperature. Gels were de-stained at room temperature in 30% methanol/7.5% acetic acid for 10 hr and imaged several times throughout the de-staining process with the ChemiDoc System (Bio-Rad). Band intensities representing the protease activity of the MMP isoforms were quantified as described above.
Enzyme-Linked Immunosorbent Assays (ELISAs)
Concentrations of MMP-2, TIMP-1, TIMP-2, and MCP-1 in CSF were measured using ELISA kits from R&D Systems according to the manufacturer's instructions (Product #DMP2F0, #DTM100, #DTM200, #DCP00, respectively). S100B levels in CSF were measured using an ELISA kit from BioVendor R&D (Product #RD192090100R). Samples were run in triplicate for MMP-2, TIMP-1, TIMP-2 and diluted 1:2, 1:25, 1:50, respectively. Samples were run in duplicate for MCP-1 and diluted 1:5. Undiluted samples were run in duplicate for S100B. Both the pro- and mature forms of MMP-2 were detected, although active MMP-2 complexes were not detected.
RESULTS
Multiplex Immunoassay Data Analysis
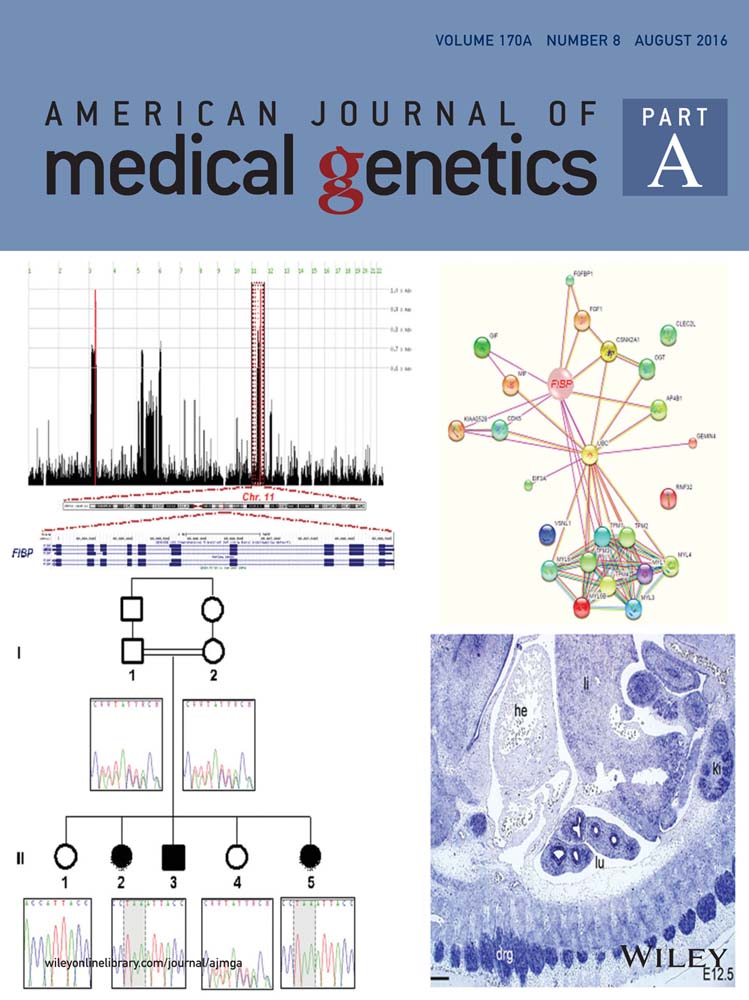
Our initial analysis of potential SLOS protein biomarkers in CSF included the quantitative measurements of 70 analytes in SLOS subjects and controls (Supplemental Table SII). From the 20 SLOS patient samples analyzed in the discovery portion of the study, the average age was 7.7 years and the gender breakdown was 13 males and 7 females. CSF DHC/total sterol ratio ranged from 0.3% to 13.6%. The analytes measured in this discovery assay included inflammatory markers, chemokines, cytokines, hormones, and growth factors. Of the 70 analytes that were measured, 14 were below the LOD for >40% of the samples and were excluded from further analysis. A list of these 14 analytes is provided (Supplemental Table SIII). Of the 56 remaining analytes, 12 analytes demonstrated significant differences between the control and the SLOS patient samples (Table I). These 12 candidate protein biomarkers were evaluated to determine if a correlation existed between protein concentration and disease severity, cholesterol, or 7-DHC concentrations in the CSF. Only three analytes had significant correlations; monocyte chemoattractant protein-1 versus 7-DHC (P = 0.04), kidney injury molecule-1 versus 7-DHC (P = 0.007) and apolipoprotein c-1 versus cholesterol (P = 0.02). The three correlation plots including Spearman's correlation (r) values are provided in Figure 1.
| Log10 concentration mean ± SD | |||
|---|---|---|---|
| Analyte | Control | SLOS | P-value |
| Monocyte chemoattractant protein-1 (pg/ml) | 2.82 ± 0.21 (n = 30) | 2.63 ± 0.11 (n = 20) | 0.0001 |
| Matrix metalloproteinase-2 (ng/ml) | 0.158 ± 0.15 (n = 30) | 0.602 ± 0.44 (n = 20) | 0.0002 |
| Connective tissue growth factor (ng/ml) | 0.225 ± 0.125 (n = 24) | 0.35 ± 0.11 (n = 20) | 0.001 |
| Calbindin (ng/ml) | 1.95 ± 0.24 (n = 25) | 2.17 ± 0.17 (n = 20) | 0.001 |
| Macrophage inflammatory protein-1-β (pg/ml) | 1.20 ± 0.35 (n = 30) | 0.953 ± 0.15 (n = 19) | 0.001 |
| Kidney injury molecule-1 (ng/ml) | −1.48 ± 0.20 (n = 27) | −1.35 ± 0.07 (n = 20) | 0.004 |
| Serum glutamic oxaloacetic transaminase (ug/ml) | −0.361 ± 0.15 (n = 30) | −0.244 ± 0.16 (n = 20) | 0.009 |
| S100-β (ng/ml) | −0.152 ± 0.32 (n = 30) | 0.048 ± 0.21 (n = 20) | 0.02 |
| Apolipoprotein C-I (ng/ml) | −1.24 ± 0.28 (n = 30) | −1.09 ± 0.17 (n = 20) | 0.02 |
| Superoxide dismutase-1 (ng/ml) | 1.81 ± 0.28 (n = 30) | 1.95 ± 0.14 (n = 20) | 0.03 |
| Transferrin (mg/dl) | -0.0242 ± 0.26 (n = 30) | 0.0999 ± 0.13 (n = 20) | 0.03 |
| Transthyretin (mg/dl) | 0.136 ± 0.12 (n = 27) | 0.073 ± 0.08 (n = 20) | 0.05 |
Of the 12 analytes with differential CSF expression in SLOS, a subset of these proteins—monocyte chemoattractant protein-1 (MCP-1), matrix metalloproteinase-2 (MMP-2), connective tissue growth factor (CTGF), and S100B are commonly associated with extracellular matrix (ECM) turnover and brain injury [Friedrichsen et al., 2003; Deshmane et al., 2009; Rosenberg, 2009; Sorci et al., 2013]. Elevated levels of MMP-2, CTGF, and S100B and decreased levels of MCP-1 were observed in SLOS patients relative to the control group. We thus elected to further explore these proteins in a validation set of CSF.
MCP-1
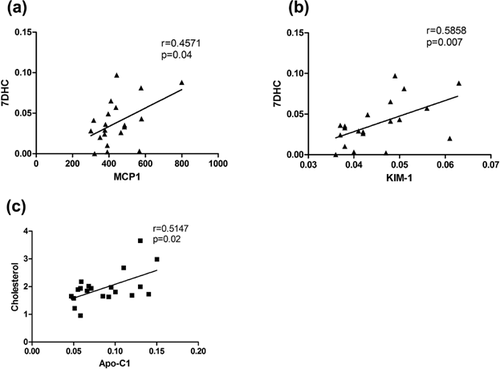
Monocyte chemoattractant protein-1 (MCP-1/CCL2) is a chemokine that is produced by multiple cell types following stimulation by cytokines, growth factors, or oxidative stress [Deshmane et al., 2009]. MCP-1 is produced by glia as well as neurons, and functions to regulate the migration of monocytes, memory T cells, natural killer cells and microglia to areas of inflammation [Deshmane et al., 2009]. Semple et al. [2010] observed elevated MCP-1 Levels in patients suffering from traumatic brain injury and confirmed a functional role for MCP-1 in modulating brain damage. MCP-1/CCL2 deficient mice showed altered cerebral cytokine levels, reduction in macrophage/microglia accumulation, and astrogliosis that led to improved recovery rates following brain injury in these mice. We observed a decrease of MCP-1 in SLOS CSF compared to control CSF (Log10 transformed concentrations (pg/ml) for control: 2.82 ± 0.21, and SLOS: 2.6 3 ± 0.11; P = 0.0001 (Table I, Fig. 2a). We sought to validate this observation by an independent ELISA using age- and gender-matched controls. Contrary to the initial results obtained by RBM we noted a trend toward elevated MCP-1 Levels in SLOS patient CSF relative to control (Fig. 2b). We performed a cell migration assay using human monocytic cells, THP1. Following exposure to media only, control CSF, or SLOS patient CSF, no difference in migratory cell number was observed between the groups (Supplemental Fig. S1).
MMPs and TIMPs
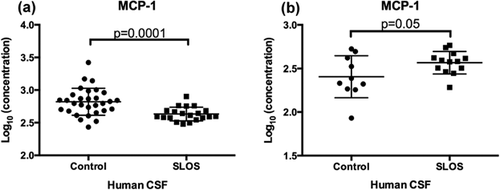
The family of matrix metalloproteinases (MMPs) consists of more than 20 zinc-dependent proteases that degrade components of the ECM and basement membranes. The family is divided into four subgroups: collagenases, gelatinases, stromelysins, and membrane-type MMPs [Rosenberg, 2009]. MMPs are secreted as inactive zymogens, which are then cleaved into active forms that regulate a variety of biological activities such as tissue remodeling, apoptosis, synaptic plasticity, angiogenesis, neurogenesis, and inflammation [Yong, 2005; Rosenberg, 2009]. Gelatinase A (MMP-2) and gelatinase B (MMP-9) have been intensively studied in the brain due to their prominent role in injury and repair [Rosenberg, 2009]. Analysis of the multiplex immunoassay data showed that MMP-2 Levels are significantly elevated in CSF from SLOS subjects when compared to controls (Log10 concentration (ng/ml): control 0.158 ± 0.15; SLOS: 0.602 ± 0.44, P = 0.0002) (Table I, Fig. 3a). We observed a similar trend in MMP-2 Levels using an independent ELISA, (Log10 concentration (ng/ml): control 1.46 ± 0.1; SLOS 1.52 ± 0.08 (Fig. 3b).
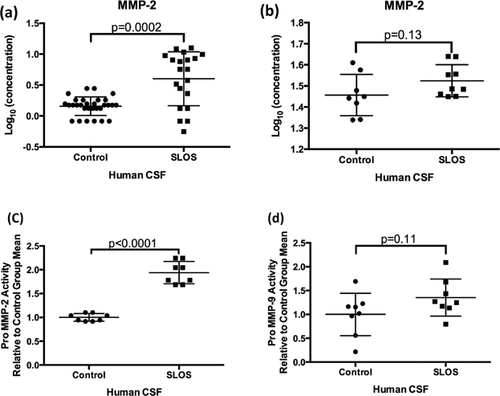
To further investigate the potential differences in the expression of MMP-2 in SLOS CSF, gelatin zymography was used to examine the relative amounts of pro- and active MMP-2. We also extended these studies to evaluate MMP-9, a closely associated family member, which was excluded from analysis of the MAP data due to the majority of the samples being below the LOD. For these studies, we used control and SLOS CSF. Strong protease bands at 92 and 72 kDa indicated the presence of proMMP-9 and proMMP-2, respectively in all samples. Quantification of band intensities showed a significant two-fold elevation of pro MMP-2 (72 kDa) activity (Fig. 3c, P < 0.0001) and no significant difference was observed for pro MMP-9 (92 kDa) activity in SLOS CSF (P = 0.11) (Fig. 3d). Most of our samples showed no quantifiable active MMP-2 band, although faint bands at 63 kDa indicated the presence of some active MMP-2 in the SLOS subjects. Active MMP-9 bands at 92 kDa were also faintly visible in all patient samples. MMP-9 Levels in CSF were undetectable by ELISA (data not shown). No changes in MMP-2 or MMP-9 gelatinolytic activity were observed in the serum of SLOS patients compared to control patients (Supplemental Fig. S2).
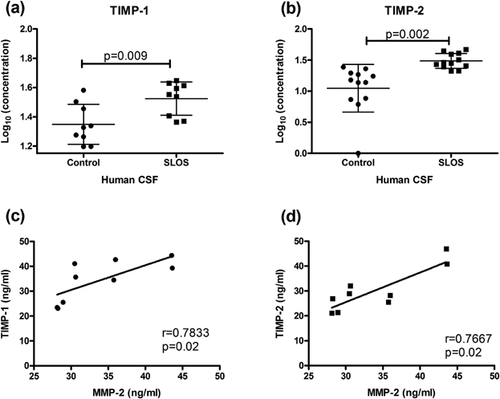
In addition to analyzing MMP-2 and MMP-9 Levels, we examined if levels of their inhibitors, TIMP-2 and TIMP-1 respectively, were altered. TIMP-1 and TIMP-2 Levels were measured in SLOS CSF and control CSF by ELISA (Fig. 4). Both TIMP-1 (Log10 (ng/ml) of control: 1.35 ± 0.14, SLOS: 1.52 ± 0.11; P = 0.01) and TIMP-2 (Log10 (ng/ml) of control: 1.05 ± 0.38, SLOS: 1.49 ± 0.12; P = 0.002) concentrations were significantly elevated in SLOS patients (Fig. 4a and b). Additionally, TIMP-1 (P = 0.02) and TIMP-2 (P = 0.02) levels had a positive correlation with MMP-2 Levels (Fig. 4c).
Connective Tissue Growth Factor (CTGF)
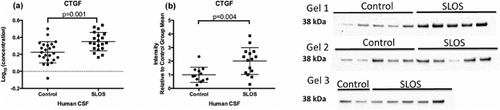
CTGF is a cysteine-rich 36–38 kDa protein secreted by multiple cell types [Friedrichsen et al., 2003]. In the CNS, CTGF appears to be an important mediator of TGF-beta function in response to brain injury and inflammation [Friedrichsen et al., 2003]. Our analysis of data from the multiplex immunoassays revealed a significant increase in CTGF levels in SLOS CSF compared to control CSF (control: Log10 0.2252 ng/ml ± 0.03, SLOS: Log10 0.3502 ng/ml ± 0.02; P = 0.001) (Table I, Fig. 5a). We validated this finding in CSF by Western blot analysis (control n = 12, SLOS n = 15; P = 0.004) (Fig. 5b). While levels of CTGF positively correlate with MMP-2 Levels in exfoliative glaucoma [Ghanem et al., 2011], we did not observe any correlation in our subjects (P = 0.81) (Supplemental Fig. S3).
S100B
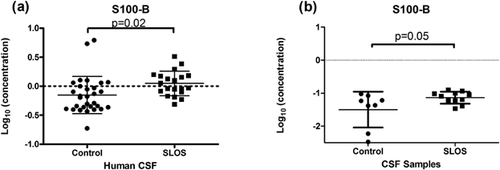
The S100B protein is a cytosolic, calcium binding protein with a variety of functions. In our study, we observed significantly elevated levels of S100B in the CSF of SLOS patients from the multiplex immunoassay data (Log10 concentration (ng/ml), SLOS: −0.152 ± 0.32, control: 0.048 ± 0.21; P = 0.02) (Table I, Fig. 6a) and validated this observation by an independent ELISA with age- and gender-matched controls (Log10 concentrations (ng/ml): control 1.50 ± 0.54 versus SLOS: −1.87 ± 0.18; P = 0.05) (Fig. 6b).
DISCUSSION
This study revealed that MCP-1 may be altered in SLOS patients. Related to SLOS biochemistry defects, oxysterols which have strong pro-inflammatory effects, including a role in up-regulating NF-kappa B, are closely associated with the production of inflammatory cytokines such as TNF-alpha, IL-1beta, VCAM-1 and MCP-1 [Vejux and Lizard, 2009]. Inflammation can lead to increased reactive oxygen species (ROS) production, which triggers further oxidation of cholesterol and oxysterols, thereby generating a detrimental self-propagating cycle [Poli et al., 2013]. S100B is a member of the S100 family of Ca2+ binding proteins that are expressed in a cell-specific fashion [Donato et al., 2013]. In the CNS, S100B is primarily expressed by astrocytes and appears to function in regeneration and repair through its ability to stimulate cell proliferation, migration, and inhibit apoptosis [Donato et al., 2013]. However, over-expression of S100B may lead to neurotoxic effects [Sedaghat and Notopoulos, 2008]. Similar to MCP-1, S100B which was found to be altered in SLOS, is also a marker of astrocyte activation [Donato et al., 2013], which can be linked to oxysterols. We observed an elevation of S100B in CSF from SLOS patients in our initial study; however validation of this finding might only indicate a minor elevation. Critical analysis of our initial data set did not reveal any outliers that may have skewed the dataset. However, the reduced number of unaffected “control” samples in our validation group may explain this result, as there appears to be greater variability in the levels of S100B present in the “normal population” (Figs. 6a and b).
MMP-2, TIMP-1, and TIMP-2 levels are elevated in the CSF of SLOS patients. The elevation of MMP-2 is largely due to increased levels of the pro-form. Pro MMP-9 also appears to be slightly increased. MMPs affect brain development through cleavage of proteins that function in synaptogenesis, synaptic plasticity, and long-term potentiation. Large CNS neurons are surrounded by proteoglycans such as brevican and the glycoprotein tenascin-R (TN-R). Cleavage of TN-R alters synaptic structure and results in learning and memory deficits [Montag-Sallaz and Montag, 2003]. Other targets of MMP include activation of cytokines and growth factors, such as the pro-inflammatory cytokine TNF-alpha, nerve growth factor, and brain derived neurotrophic factor which regulate neuronal survival, development, and synaptic plasticity [Ethell and Ethell, 2007]. Correlation analysis suggests that elevated MMP-2 levels in SLOS patients and elevated TIMP levels such as TIMP-1 and TIMP-2 may inhibit MMP-2 and be responsible for regulating MMP proteolytic activity. It has been shown that over-expression of superoxide dismutase proteins in a mouse model results in increased MMPs upon cold injury induction suggesting a link between these two families of proteins [Morita-Fujimura et al., 2000]. The family of tissue inhibitor of metalloproteinases (TIMPs) includes four proteins that inhibit MMP activity through the formation of non-covalent complexes [Lorenzl et al., 2003]. TIMPs are involved in a variety of biological activities including pro-apoptotic and anti-apoptotic processes, cellular interactions, ECM remodeling, brain injury and repair [Lorenzl et al., 2003; Rosenberg, 2009]. These proteins, together with CTGF and S100B that were found to be elevated as well, constitute a set of CSF protein biomarkers novel to SLOS. Proteins altered in the CSF will be useful in monitoring efficacy of potential therapies for SLOS and may also lead to a better understanding of the pathophysiology underlying SLOS.
The elevation of a subset of proteins in the CSF may be linked to increased oxysterol levels in SLOS patients, both in the CNS and systemically. Oxysterols are highly reactive biochemical compounds. Their oxygen groups allow them to cross lipophilic membranes, including the blood-brain barrier, and therefore impact brain pathophysiology in a significant manner [Bjorkhem et al., 2009]. Studies have demonstrated that the accumulation of 7-DHC in SLOS may be particularly detrimental. The large lipid peroxidation rate constant for 7-DHC indicates that it is highly capable of triggering a free radical chain reaction that can lead to the production of ROS and the formation of additional oxysterols in tissues [Korade et al., 2010]. In mouse peritoneal macrophages and human pro-monocytic cells, oxysterols have been shown to upregulate MMP-9 by inducing ROS production through the activation of Nox2, although TIMP-1 and TIMP-2 levels were unaffected [Gargiulo et al., 2011]. Furthermore, ROS were shown to independently upregulate pro-fibrotic and matrix remodeling proteins including CTGF and MMP-2 in mitral valves from humans with myxomatous mitral valve disease [Hagler et al., 2013]. Consistent with a hypothesis that oxidative stress is persistent in SLOS patients, our survey includes an increase of the protein superoxide dismutase-1 in SLOS patients. Correlations between oxidative stress markers and matrix metalloproteinase activity in coronary artery disease have also been reported [Kameda et al., 2003]. The increase in MMP activity observed in this study supports a role in oxidative stress. Additionally, the positive correlation of MMP activity and TIMP protein levels provide a means to evaluate these patients. Finally, TIMP proteins have been reported to be involved in modulating neurite outgrowth [Ould-yahoui et al., 2009]. Previous studies from our laboratory have reported on lengthened dendrites and axons in the SLOS mouse model [Jiang et al., 2010] which is associated with the activation of Rho GTPases.
The enclosed report provides a group of protein biomarkers related to SLOS, specifically focused on the CSF. While sample groups tend to be small when studying rare diseases, these findings are important in the development of therapeutic options and obtaining a better understanding the disease. While MCP-1, MMPs, TIMPs, CTGF, and S100B are distinct proteins, their altered levels in SLOS patient CSF relative to unaffected “control” CSF support the involvement of brain injury and repair in contributing to the pathophysiology of SLOS. Taken together, these data support defects in the extracellular matrix and remodeling of the brain in SLOS. Future studies will be geared towards extending this understanding of SLOS and providing optimal treatment options for SLOS patients.
ACKNOWLEDGMENTS
This work has been supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, and DHHS. Financial support from the Smith–Lemli–Opitz/RSH Foundation is also acknowledged. The authors would like to thank the patients and their families for their support and participation.