14q13 distal microdeletion encompassing NKX2-1 and PAX9: Patient report and refinement of the associated phenotype
Abstract
Chromosome 14q11-q22 deletion syndrome (OMIM 613457) is a rare genomic disorder whose associated phenotype is heterogeneous, depending on the size, and, mostly, on the deleted region. We report the clinical and molecular characterization of a female newborn, whose phenotype was characterized by poor growth, dysmorphic facial features, subclinical hypothyroidism, and mild reduction of CD3CD8 Lymphocytes with increased CD4/CD8 ratio. By array-CGH, we identified a 4.08 de novo interstitial deletion of the 14q13.2q21.1 region, which includes 16 OMIM genes.Our patient phenotype is compared with other published cases, for a better classification of the 14q11-q22 deletion syndrome. We demonstrated that the 14q13.2q21.1 deletion, which encompasses NKX2-1, but not FOXG1 gene and HPE8 region, identifies a well defined, more benign, microdeletion syndrome. This report confirms that an early identification with accurate characterization of the genomic disorders is of great relevance, enabling proper genetic counseling of the reproductive risk, as well as disease prognosis, and patient management. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Chromosome 14q11-q22 deletion syndrome (OMIM 613457) is a rare genomic disorder, caused by an interstitial deletion of the long arm of chromosome 14 with variable breakpoints. The deletion has been described as a contiguous gene syndrome in several reports [Shapira et al., 1994; Kamnasaran et al., 2001; Caliebe et al., 2011; Fonseca et al., 2012; Piccione et al., 2012; Santen et al., 2012]: FOXG1, NKX2-1, and PAX9 have been identified as the candidate dosage-sensitive key genes of clinical significance, while the role of other genes (i.e., GARNL1/TULIP1, NKX2-8, SLC25A21) is still debated.
Based on literature reports, no recurrent breakpoints are present: the 14q deletion associated phenotype seems heterogeneous, depending on the size, and, mostly, on the deleted region. When the deletion is proximal [chr14(GRCh37):28,000,000–35,000,000], the phenotype is severely compromised with intellectual disability, CNS malformations (i.e., agenesis of the corpus callosum, CCA), and poor prognosis. The distal 14q deletion [chr14(GRCh37):35,000,000–40,000,000] seems associated with a milder phenotype: intelligence is normal or slightly impaired; distinctive clinical features are choreoathetosis and congenital hypothyroidism, with or without pulmonary dysfunction (CAHTP, OMIM 610978) [Santen et al., 2012].
We report a new patient with 14q13 distal microdeletion syndrome. The chromosome breakpoint was estimated as a 4.08 Mb sized deletion [arr14q13.2q21.1 (35,443,407–39,527,387) × 1] by array CGH, performed soon after birth because of craniofacial dysmorphisms. Our case confirms a close relationships between clinical findings and 14q deleted region, underlining the need of early genetic diagnosis for better counseling, clinical assessment, and follow up.
CLINICAL REPORT
The proband is a female newborn, of Caucasian non-consanguineous parents: 38-year-old father and a 37-year-old mother. Family history is unremarkable; no neurological and/or psychiatric disorders have been reported.
She was born at 39 weeks by cesarean section because of fetal distress, after an uneventful pregnancy. Apgar score was 7/8, birth weight 2,620 g (3rd centile), height 46 cm (3rd centile), and head circumference 33.5 cm (>10th centile).
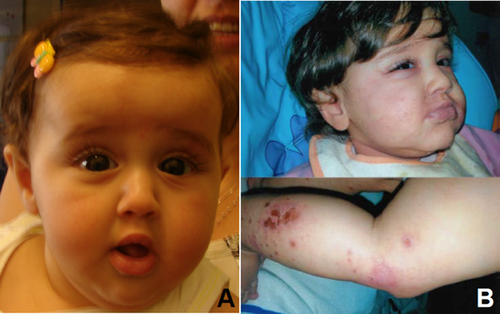
Soon after birth, she presented significant nasal obstruction and a Mayo cannula was applied. Craniofacial dysmorphisms were present (Fig. 1A): broad forehead with frontal bossing, large ears, down-slanted eyes, depressed and broad nasal bridge, flat nasal root, deviated nasal septum, stenosis of the left choana, small mouth, high arched palate, and central maxillary hypertrophy. Brain MRI, renal ultrasonography, echocardiography, ophthalmologic- and ORL examinations were all normal.
Genetic analysis revealed a de novo 14q13.2q21.1 interstitial microdeletion. Accordingly a clinical follow up was started. At 10 months of life, psychomotor evaluation was normal and auxological parameters were: weight 7,500 g (10th centile), height 66 cm (<3rd centile), and head circumference 43.5 cm (>10th centile). She suffered from recurrent rhinitis. A subclinical hypothyroidism was diagnosed: TSH 19.7 mIU/L (normal 0.36–3.74), fT4 1.03 ng/dl (normal 0.76–1.46), fT3 4.6 pg/ml (normal 1.7–5.2) together with a mild reduction of CD3CD8 Lymphocytes with an increased CD4/CD8 ratio (3.9, normal ratio 1.4–1.8). Complete blood cell counts, liver and renal function tests, blood glucose, IgA, IgG, and IgM levels were all within normal limits.
At subsequent follow up, at age of 15 months, no psychomotor development delay was present, weight and head circumference were at 25th centile, while height <3rd centile. Only 4 teeth had erupted, with a single upper central incisor. Because of still elevated TSH, according to the endocrinologist tyroxine supplementation was started. She had a serious staphylococcal infection at the age of 1 year (Fig. 1B) for which hospitalization and intravenous antibiotic therapy were required.
GENETIC INVESTIGATION
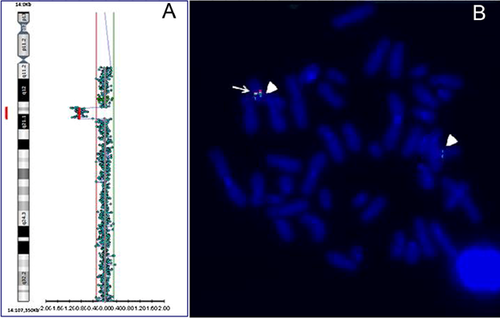
Cytogenetic analysis, performed on QFQ-banded metaphases (550-band level), showed a normal female karyotype (46,XX). Genomic DNA was extracted from peripheral blood. DNA concentration was measured by fluorimeter (Amersham, Piscataway, NJ) using the Hoechst reagent and adjusted to 400 ng/ml. Array-CGH analysis was performed using the Cytochip oligo ISCA 4 × 44 K (TechnoGenetics Srl, Italy). This array is composed of 60-mer oligonucleotides spaced at about 75 Kb density across the genome. Labeling and hybridization were carried out following the manufacturer's protocol. Slides were scanned on the InnoScan 710 (Innopsys), and analyzed using the BlueFuse Multi v3.1 (Bluegnome). The analysis revealed an interstitial deletion of the long arm of chromosome 14, with a loss in copy number at the 14q13.2q21.1 region [chr14:35,443,407–39,527,387 (GRCh37), size 4,083,981 bp] (Fig. 2A). Result of array CGH analysis were confirmed by FISH. BAC probes RP11-561B11 (proximal 14q13.2), RP11-481F8, RP11-537P22, and RP11-506K19 (distal 14q21.1) (Bluegnome), mapping in the deleted region, produced hybridization signals only on the normal chromosome 14 (Fig. 2B). The deletion was not present in the parents. The 4.08 Mb deleted region contained 16 OMIM genes: SRP54, KIAA0391 (2), PSMA6, NFKBIA, INSM2, GARNL1, MBIP, NKX2-1, NKX2-8, PAX9, SLC25A21, MIPOL1, FOXA1, SSTR1, and SEC23A. The final karyotype of the patient was designed as (ISCN 2013): 46,XX.arr 14q13.2q21.1(35,443,407–39,527,387) × 1 dn.
DISCUSSION
We report the clinical and molecular characterization of a newborn female with a mild phenotype, characterized by dysmorphic facial features, and poor fetal growth (weight and height ≤ 3rd centile). Using array-CGH, we identified a 4.08 de novo interstitial deletion of the 14q13.2q21.1 region, which includes 16 OMIM genes.
The comparative evaluation of our case with other 14q deletion reported cases is not easy: the size and breakpoints of the deleted regions are highly variable, with different phenotypes. Santen et al. [2012], presenting the molecular karyotyping and the phenotypic description of seven patients with partially overlapping 14q13 deletions, assumes that the impact on the phenotype of the deletion of the 14q13 region is mainly due to haploinsufficiency of three genes: FOXG1, NKX2-1, and PAX9. When FOXG1 is deleted, the development is severely delayed and the prognosis is poor, if haploinsufficiency is limited to NXKX2-1 and PAX9, the phenotype is milder with quite normal psychomotor development [Santen et al., 2012].
We have found only two patients with a deletion similar to our case: patient 1, reported by Santen et al. [2012], and patient 3, described by Dale et al. [2012], while all other cases showed larger or smaller deletions [Shapira et al., 1994; Kamnasaran et al., 2001; Caliebe et al., 2011; Torgyekes et al., 2011; Fonseca et al., 2012; Piccione et al., 2012; Santen et al., 2012; Hayashi et al., 2015]. The two patients, respectively 7 and 1.5 years old, have hypothyroidism, movement disorders (chorea), hypo/oligodontia, and normal growth. Mild developmental delay was present with joint laxity and gait disturbances in the patient described by Dale et al. [2012] (patient 3), while motor delay was reported in the Santen et al. case [2012] (patient 1). In both cases, no lung involvement was reported [Dale et al., 2012; Santen et al., 2012]; this data is of prognostic relevance because pulmonary disease is responsible for significant mortality in patients with NKX2-1 anomalies [Carré et al., 2009].
These reports, together with our case, suggest that when the 14q13 deletion is distal, the phenotype is milder, more benign, mainly due to the haploinsufficiency of the NKX2-1, PAX9, and, probably, NFKBIA genes.
The most important pathogenetic role is that of NKX2-1 gene (or TITF1, thyroid transcription factor 1, OMIM 600635), which encodes for a NK-2 family transcription factor, expressed in thyroid gland, lung, and the forebrain. In the lung, NKX2-1 contributes to branching of epithelium, alveolarization and surfactant production [Barnett et al., 2012], while, in the brain, it is involved in neuronal migration, and in the development of the basal forebrain, pituitary, hypothalamus, and basal ganglia [Goulburn et al., 2011]. NKX2-1 mutations are associated with a rare disease (CAHTP, OMIM 610978), characterized by a highly variable penetrance and expressivity, with manifestations ranging from benign hereditary chorea (BHC), the hallmark of NKX2.1-related disorders, to choreoathetosis, congenital hypothyroidism, and pulmonary disease (neonatal respiratory distress and/or interstitial lung disease).
According to Williamson et al. [2014], brain and/or thyroid involvement is very common, while lung is involved in less than 50% of cases. This phenotypic variability can be explained considering that NKX2-1 is an haplosensitive gene (DECIPHER HI% of 1.49): the positional effect of one or more deleted genes [Lupski and Stankiewicz, 2005], the tissue-specific gene expression regulation, as well as the extent of the deletion and the role of putative modifier genes, not present in the critical region, all contribute to the phenotype [Barnett et al., 2012]. In our case, the respiratory distress at birth was due to the deviated nasal septum with left choana stenosis. The subclinical hypothyroidism could be due to NKX2-1 deletion. At last examination psychomotor development was normal, but our patient is still too young (15 months) to exclude the possible onset of movement disorders (with a median onset age of 2 years), as well as of mild/moderate intellectual disability.
PAX9 is a transcription factor involved in the odontogenesis and craniofacial skeletogenesis, especially of the palate. Deletions or mutations of PAX9 have been previously described in patients with oligodontia, including families with autosomal dominant inheritance [Stockton et al., 2000; Guala et al., 2008]. Based on literature findings, the PAX9-related oligodontia is associated to the agenesis of permanent maxillary and mandibular second molars, while the primary dentition is often normal [Haldeman-Englert et al., 2012]. Recently, Zhou et al. [] have also demonstrated that Pax9 regulates a molecular network, involving the Bmp4, Fgf10, Shh signaling and the Osr2 transcription pathways, to control palate morphogenesis. At 15 months of age, our patient shows a very tight and arched palate with only four incisor teeth erupted, so that oligodontia is a very likely possibility. The presence of a single upper central incisor could indicate a midline development defect, due to PAX9 deletion, as suggested by the stenosis of the left choana [Hall, 2006].
Finally, the Nuclear Factor of Kappa light chain gene enhancer in B cells Inhibitor (NFKBIA) encodes for a master transcription factor required for normal activation of the immune response. Impaired NFKB signaling, due to mutations of this gene, causes an anhidrotic ectodermal dysplasia with T-immunodeficiency (EDA-ID, OMIM 612132), firstly described by Courtois et al. [2003]. However, the genotype–phenotype correlation are still not clear: Janssen et al. [2004] have described two related subjects with the same NFKBIA mutation, but completely different clinical syndromes. In our patient, NFKBIA haploinsufficiency could be responsible of recurrent rhinitis, severe staphylococcal infection, and mild reduction in CD3/CD8 Lymphocytes with an increased CD4/CD8 ratio.
The role of other genes is still debated (i.e., GARNL1/TULIP1, NKX2-8, SLC25A21). For example, SLC25A21, the solute carrier family 25 (mitochondrial oxodicarboxylate carrier), was previously described as a possible cause of 2-oxoadipate acidemia, an inborn error of metabolism of lysine, tryptophan and hydroxylysine, associated to mental delay, hypotonia, and seizures [Fiermonte et al., 2001]. Meyertholen et al. [2012] suggested an association of 14q13.3 deletion, involving the SLC25A21, with a familial synpolydactyly, thorough a possible interaction between SLC25A21, PAX9, and MIPOL1. MIPOL1 is a candidate gene for mirror-image polydactyly [Kondoh et al., 2002], but, until now, the clinical significance of copy number variations of this gene is unclear.
In conclusion, our report shows that, despite the extreme rarity, the 14q13 distal deletion encompassing NKX2-1, but not FOXG1 gene and/or HPE8 region, identifies a well defined, more benign, microdeletion syndrome: except for growth retardation, all the signs and symptoms of our patient can be explained on the basis of the deleted genes. An accurate nosological classification is of great help for the patient and the family. It allows to make a proper genetic counseling about the reproductive risk, and the prognosis, enabling to reassure the family about the recurrence, the severity of phenotype, and the possible occurrence of associated clinical manifestations (i.e., neurological movement disorders). In addition, an appropriate targeted follow up can help to avoid unnecessary exams, often expensive and/or invasive, with the possibility of early diagnosis and prompt medical therapy, that is, for thyroid abnormalities, or respiratory infections due to the association of the deletion with chronic interstitial lung disease.
ACKNOWLEDGMENTS
We are grateful to the family who participated in this study. We thank C. Nanna, E. Tangari, and P. Zambetti for technical assistance.