A second family with CATSHL syndrome: Confirmatory report of another unique FGFR3 syndrome
Abstract
Here we describe the second reported family with the CATSHL syndrome, a condition resulting from a unique mutation in the fibroblast growth factor receptor 3 (FGFR3) gene. Our family confirms the consistent and unique phenotype of this condition, and the specificity of the mutation in FGFR3. The CATSHL syndrome appears to be an autosomal dominant disorder with full penetrance. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
In 1996, Toydemir et al. described a multigenerational family some members of whom had a unique phenotype, which was the result of a novel mutation in the fibroblast growth factor receptor 3 (FGFR3) gene. The disorder was quite distinct from those produced by other FGFR3 mutations. The major features of the condition included camptodactyly, tall stature, and hearing loss, and Toydemir et al. [1996] denoted the condition as the CATSHL syndrome. Other less consistent findings included microcephaly, kyphoscoliosis, and intellectual disability. The CATSHL syndrome appears to be an autosomal dominant disorder produced a c.1915G>A transition in exon 15 of the FGFR3 gene resulting in a substitution of a histidine for a highly conserved arginine residue (pR621H) in the kinase domain of FGFR3. This substitution induces a loss of function of the gene. Since Toydemir et al. published their article, there have been no other reported individuals or families with this condition or the same FGFR3 mutation.
CLINICAL REPORT
Our proband (IV-1, Fig. 1) was referred to our genetics center in Indianapolis at age 16 years for the genetic evaluation of her sensorineural hearing loss. Her past medical history indicated a normal pregnancy and perinatal period. Birth was at 38 weeks gestation with a birth weight of 2.358 kg (5th centile) and a length of 53 cm (97th centile). Her early childhood was unremarkable except for the development of multiple finger contractures beginning at age 3 years, which have gotten worse with time. At the age of 4, she was found to have an estimated 34% sensorineural hearing loss. By age 16, her hearing loss had advanced to 58% of normal. Since age 4 our patient had been using hearing aids.
Also at some time during her childhood, she developed chronic joint pain involving the hips and lower back. Because of her contractures and the joint pain at the age of 6, she was evaluated at Chicago's Shriners Hospital for Children. Here, her digital anomalies were thought to be post-traumatic. An attempt to surgically correct the contractures there was unsuccessful. She also was found to have scoliosis. A head MRI at age 12 was within normal limits (Fig. 2).
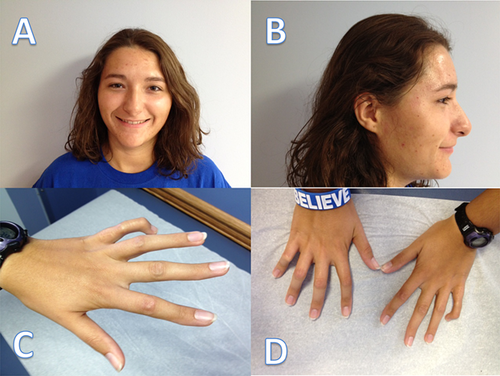
When we saw her at the age of 16, her past physical, intellectual, and social development had been normal. At the ages of 8 and 10, she had experienced two tibial stress fractures. She also had surgery to correct misalignment of the right hip. On physical exam, her height was 170 cm (90th centile), weight was 74.3 kg (90–95th centile), and OFC was 52 cm (−2 SD). In addition, she had superficial telangiectasia over the nose, long, thin, and contracted fingers, mild scoliosis, genu valgum, and a leg length discrepancy of 1.6 cm with the left being longer than the right. Radiologic images revealed a right coxa valga of the right femoral neck, the head of which was laterally displaced with 30% uncovering. The left femoral head was medially displaced. Sequencing of the fibroblast growth factor receptor 3 (FGFR3) gene revealed a c.1915G>A (pR621H) transition in exon 15 of the FGFR3.
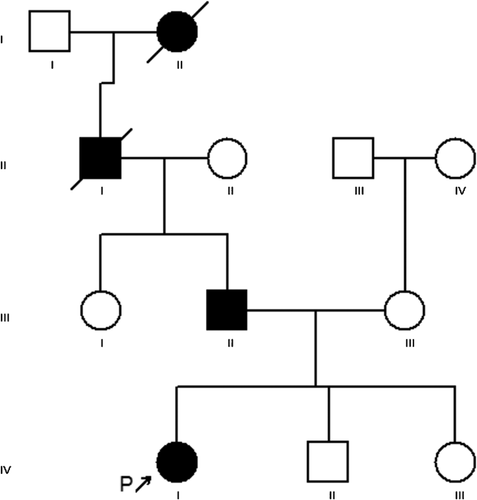
Family history was positive. The proband's father (III-2, Fig. 1) has progressive hearing loss, three ruptured vertebral discs, and camptodactyly of fingers and toes. He also is tall with a height of 200.7 cm (>97th centile). By history, Proband's paternal grandfather (II-1, Fig. 1) has hearing loss and camptodactyly (limited information is known on this individual). The ethnic background of the proband is French, German, Native American, and Swiss. There is no family history of other birth defects or genetic conditions.
DISCUSSION
Reported here are four affected family members in four generations with the physical findings consistent with CATSHL syndrome. Our diagnosis of this condition was done by searching the literature for the proband's physical findings and sequencing of the FGFR3 gene. This family confirms the consistent and unique phenotype of CATSHL syndrome and the specificity of the mutation in FGFR3. Based on vertical transmission and male-to-male transition of the syndrome in our family and the previously reported family [Toydemir et al., 1996], the condition appears to be an autosomal dominant disorder with full penetrance (Table I).
| Features | Percentage of affected individuals Toydemir et al. [1996] | Escobar et al. (Current report) |
|---|---|---|
| Tall stature | 93 | 4/4 |
| Camptodactyly of hands and/or feet | 90 | 4/4 |
| Bilateral sensorineural hearing loss | 85 | 4/4 |
| Developmental delay/intellectual disability | 60 | 0/3 |
| Microcephaly | Several | 0/3 |
| High palate | Not stated | 2/4 |
| Pectus excavatum | Not stated | 0/4 |
| Scoliosis/kyphoscoliosis | Not stated | 2/4 |
| Tall vertebral bodies | Not stated | 0/3 |
| Vertebral disc rupture | Not stated | 2/4 |
| Coxa valga/broad femoral metaphyses | Not stated | 2/4 |
| Bilateral genu valgum | Not stated | 3/4 |
| Osteochondromas | Not stated | 0/3 |
| Leukemia | Not stated | 1/4 |
CATSHL syndrome was first described and named by Toydemir et al. in 1996. In their publication, they described a multigenerational family with 27 living affected members, all of whom possessed a unique phenotype. Their condition was a result of a novel fibroblast growth factor receptor 3 (FGFR3) gene mutation. The disorder was quite distinct from that produced by other FGFR3 mutations. Major features included camptodactyly of the hand and/or feet, tall stature, and hearing loss, and the authors denoted this condition as the CATSHL syndrome. Other less consistent findings included microcephaly, a high palate, pectus excavatum, scoliosis/kyphoscoliosis, tall vertebral bodies with irregular borders, broad femoral metaphyses, osteochondromas, and intellectual disabilities. The syndrome was produced by a c.1915G>A transition in exon 15 of the FGFR3 gene resulting in a substitution of a histidine for a highly conserved arginine residue (pR621H) in the kinase domain of FGFR3. This substitution induces a loss of function of the gene. This change converts a codon from alanine (GCC) to a codon for threonine (ACC) in a serine-threonine/tyrosine protein kinase catalytic domain. As the disorder was published by Toydemir et al. [1996], there have been no other published reports of this condition or the c.1915G>A mutation in FGFR3.
Hearing loss in CATSHL syndrome appears to be sensorineural, congenital, and progressive in all affected individuals in our family. The hearing loss may be related to the role of FGFR3 in supporting cells of the organ of Corti [Pirvola et al., 1995]. Similar audiologic findings have been observed in Muenke syndrome, another FGFR3 condition [Mansour et al., 2009]. The developmental difficulties and intellectual disabilities in CATSHL appear to be variable and only seen in 60% affected individuals in the family reported by Toydemir et al. [1996]. None of the affected family members has intellectual disability although each possessed the major features of CATSHL syndrome (camptodactyly, tall stature, and hearing loss). The mechanism leading to the intellectual disability in some individuals is unknown. There were no structural abnormalities seen in the MRI in our proband. In the family reported here, one affected individuals (II-I) died from leukemia (type unknown). We do not know if this cancer is related to him having CATSHL syndrome. However, chronic lymphocytic leukemia has been described in translocations involving FGFR3 [Geller et al., 2014].
Features not reported in Toydemir et al.'s family [1996] but present in our family included vertebral disc rupture (2), coxa valga/broad femoral metaphyses (2), genu valgum (2), and leukemia (1). If these features truly are associated with this condition, further reports of affected families should confirm it.
Fibroblast growth factors is a family of polypeptide growth factors involved in mitogenesis, angiogenesis, and wound healing [Keegan et al., 1991]. Their cell-surface receptors are fibroblast growth factor receptors 1, 2, and 3, which are involved in intracellular signaling. The FGFR3 gene contains an extracellular domain with either two or three immunoglobulin like domains, a transmembrane domain, and a cytoplasmic tyrosine kinase domain [Keegan et al., 1991]. Mutations in this gene can result in a number of different syndromes including achondroplasia, Crouzon syndrome with acanthosis nigricans, epidermal nevus, hypochondroplasia, LAAD syndrome, Muenke syndrome, and thanatophoric dysplasia type I and II [Muenke et al., 1997; Cappellen et al., 1999; Horton and Lunstrum, 2002; Lievens and Liboi, 2003]. In addition to these syndromes, mutations in FGFR3 have been associated with human bladder and cervical cancer [Cappellen et al., 1999], chronic lymphocytic leukemia, colorectal cancer, and spermatocytic seminoma [Geller et al., 2014], The potential of FGFR3 changes to induce multiple unique syndromes and cancers suggest the high importance of FGFR3 in a variety of developmental processes.
It is likely that the CATSHL syndrome is under recognized due to the syndrome's very different phenotype when compared to the other FGFR3-related disorders. The family reported here confirms not only the existence of the CATSHL syndrome's unique phenotype but also likely expands the condition's phenotype. The phenotype is very recognizable and should be considered any time the triad of camptodactyly, tall stature, and hearing loss is ascertained in an individual.