Macrodactyly in tuberous sclerosis complex: Case report and review of the literature
Abstract
Macrodactyly in the context of tuberous sclerosis complex (TSC) is a known but rare manifestation. We report the case of a boy diagnosed with TSC at 2 years and 4 months of age, presenting with bilateral macrodactyly of the first three fingers of both hands, with underlying radiographic changes, in whom molecular analysis identified a frameshift mutation on the TSC1 gene (encoding hamartin), leading to a premature stop codon. We reviewed the literature for reported cases of TSC patients with the same manifestation. In four of 14 patients, including ours, macrodactyly caused some type of joint limitation or flexion deformity, thus contradicting the established idea that this is a finding without clinical significance. Our patient is, to our knowledge, the first reported to have clear bilateral involvement. We briefly discuss the underlying mechanism for this phenomenon, which has yet to be fully elucidated, although somatic mosaicism for loss of heterozygosity at TSC loci is a plausible explanation. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
We report a child fulfilling clinical diagnostic criteria for tuberous sclerosis complex (TSC), confirmed by molecular study of TSC1 gene, with bilateral macrodactyly of the first three fingers of both hands and underlying radiographic changes. We review the published literature for other reports of this rare finding in TSC and discuss the possible mechanism for this phenomenon.
CLINICAL REPORT
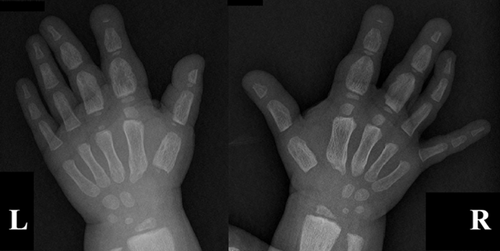
We report the case of a young boy, the first child of healthy, non-consanguineous parents, with no family history of TSC, first observed in our clinic at approximately 2 years and 4 months of age for bilateral macrodactyly of the hands and suspected TSC. He was born at 37 weeks of gestation by spontaneous vaginal delivery, after a normal pregnancy. Birth and perinatal period were uneventful. Somatometric parameters and developmental milestones were within normal limits (occipital frontal circumference on the 25th centile and weight and height on the 5th centiles). At 14 months of age, he experienced his first absence seizures. Also at this age, his parents noticed hand asymmetry with bilateral macrodactyly of the first finger of both hands and of the second and third fingers of the right hand. They also reported flexion limitation of the right second finger. There was no history of prior trauma and no other joint limitation. He later also developed progressive flexion limitation of the other involved fingers and the macrodactyly extended to the second and third fingers of his left hand (Fig. 1). Hand X-ray performed at 20 months showed irregular periosteal thickening of the metacarpal bones and phalanges of the right first three fingers, with global increase in width; the left hand showed similar, but less pronounced, changes (Fig. 2). Soft tissue ultrasound revealed diffuse increase of subcutaneous tissue, particularly on the second and third fingers of the right hand, without any evidence of focal solid or cystic lesions or tendinous lesion.
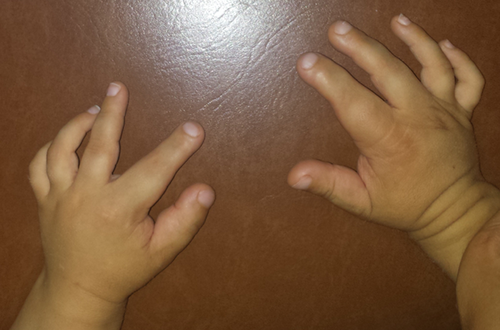
When he was first observed in our clinic, he had eight hypomelanotic macules, some with an “ash-leaf” appearance, distributed throughout the body, mainly on the thighs. He also had a shagreen patch on his left forearm. He showed no other characteristic skin lesions associated with TSC. Besides the macrodactyly, he presented thickening and hyperpigmentation of the skin overlying the affected areas. There were no facial dismorphic features. Echocardiogram revealed a rhabdomyoma on the right ventricle, with no hemodynamic consequence. Abdominal and renal ultrasounds were normal, as well as ophthalmologic examination. His parents showed no signs of TSC on the physical assessment. Cranial computed tomography (CT) performed at approximately 2 years and 3 months showed multiple subependymal punctiform hyperdense calcified nodules and hyperdense cortical-subcortical areas more prominent on the left hemisphere, in relation with possible brain tubers. He is currently medicated with carbamazepine and vigabatrin, with decreased frequency and improvement in seizure control. Thus, the clinical diagnosis of TSC was established by the presence of five major criteria [Northrup and Krueger, 2013]. TSC1 gene sequencing revealed a heterozygous point deletion at residue 1425 of exon 14 (c.1425del), causing a frameshift and leading to a premature stop codon (p.Asp476Ilefs*56). This mutation has not previously been reported in mutations databases such as LOVD (http://www.LOVD.nl/TSC1) or HGMD (http://www.hgmd.cf.ac.uk), nor in the population variants catalog ExAC (http://exac.broadinstitute.org) as of the writing of this paper. However, given its effect in a patient fulfilling the diagnostic criteria for TSC, we feel there is sufficient evidence to point to its pathogenicity. TSC2 gene analysis by sequencing and multiplex ligation-dependent probe amplification (MLPA) did not detect any point mutations or gene rearrangements. Screening for the same TSC1 mutation in his mother was negative; the father was not available for testing.
Our patient was diagnosed at 3 years and 6 months of age with speech impairment and currently needs speech and occupational therapies; his psychomotor development is otherwise normal.
DISCUSSION
Tuberous sclerosis complex is a genetic disease characterized by the growth of hamartomatous tumors, usually benign, in multiple organ systems of the body, including the brain, skin, kidneys, lungs, and heart. It is caused by inactivating mutations in TSC1 or TSC2, tumor suppressor genes located respectively on chromosomes 9q34.3 and 16p13.3 and encoding the proteins hamartin and tuberin. [Northup et al., 2010] Hamartin and tuberin form a complex (TSC1-TSC2 complex) that inhibits the activity of mammalian target of rapamycin complex 1 (mTORC1). mTORC1 is a serine/threonine kinase, member of the phosphoinositide 3-kinase-(PI3K)-related-kinase family, which is involved in the regulation of cell metabolism, proliferation, differentiation, autophagy and immune response; upon activation in the presence of growth factors, mTORC1 has an anabolic role in protein, lipid and nucleotide synthesis [Wataya-Kaneda, 2015]. Consequently, the interaction of TSC1-TSC2 complex leads to inhibition of cell growth. On the other hand, TSC1-TSC2 complex also stimulates the activity of mammalian target of rapamycin complex 2 (mTORC2), which has been showed to regulate cell survival and actin and cytokeratin organization but whose role is not yet completely understood [Wataya-Kaneda, 2015]. Cells deficient in TSC1-TSC2 complex thus show increased activity of mTORC1, stimulating cell growth, while also showing diminished activity of mTORC2. It has been proposed that this might contribute to the benign nature of the tumors in TSC, although the role of mTORC2 must be more clearly elucidated to verify the validity of this hypothesis [Huang et al., 2009].
The occurrence of macrodactyly in the context of TSC is a rare manifestation which has been previously described, although it is not included as a major or minor feature for the clinical diagnosis according to the updated diagnostic criteria recently agreed upon at the International Tuberous Sclerosis Complex Consensus Conference held in 2012 [Northrup and Krueger, 2013].
We conducted a review of the published literature regarding the presentation of macrodactyly and/or limb hyperplasia associated with TSC. We searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed) for the keywords “tuberous sclerosis” plus “macrodactyly” or “hypertrophy” or “gigantism,” obtaining the total number of 13 publications. The characteristics of those patients, as well as ours, are summarized in Table I. In most, the digits affected are the first, second and/or third fingers of the hand. This finding may be due to a reporting bias, given the small number of patients. All patients, except ours, show only unilateral involvement at physical examination, although one had bilateral changes on X-ray [Sahoo et al., 2000]. Four of fourteen patients, including ours [Hall, 1943; Sahoo et al., 2000; Sharma et al., 2011], had some type of joint limitation or flexion deformity, thus contradicting the idea that this is a finding without clinical significance.
| Features | Our patient | Sasongko et al. [2014] | Sharma et al. [2012] | Sharma et al. [2011] | Tung and Shih [2009] | Ghali [2001] | Sahoo et al. [2000] | Lustberg et al. [1999] | Norman-Taylor and Mayou [1994] | Reddy et al. [1992] | Colamaria et al. [1988] | Ortonneet al. [1982] | Reed et al. [1963] | Hall [1943] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, sex | 2 y 4 m, M | 10 y, F | New born, M | 10 y, M | 11 y, M | 8 y, M | 15 y, F | 23 y, F | 6 y, F | 18 y, M | 6 y, M | 30 y, M | NI | 17 y, M |
| Age at presentation | 14 m | 5 y | At birth | 9 m | NI | At birth | NI | NI | NI | 10 y | At birth | Infancy | NI | 3 y |
| Fingers affected | Hand, D1-3 | Hand, right D1-2 | Right lower limb; hand, right D1-5 | Hand, right D2-3 | Hand, right D1 | Foot, left D2 | Hand, left D2-3 | Hand, right D3 | Hand, right D3 | Left lower limb | Hand, right D1-2 | Left arm; hand, left D1-2 | Hand, right D3 | Hand, right D1-3 |
| Laterality | Bilateral | Unilateral | Unilateral | Unilateral | Unilateral | Unilateral | Unilateral (with bilateral radiological) | Unilateral | Unilateral | Unilateral | Unilateral | Unilateral | Unilateral | Unilateral |
| Skin changes | + (hyperpigmen-tation, thickening) | NI | + (thickening, loose, erythema) | + (hyperpigmen-tation, thickening) | − | NI | + (hyperpigmen-tation, thickening) | NI | − | + (thickening, inelasticity, dryness and wrinkling) | − | − | NI | + (thickening) |
| Mobility affected/flexion deformity | + | NI | NI | + | NI | NI | + | NI | − | NI | NI | NI | NI | + |
| X-ray | ||||||||||||||
| Increased volume/width | + | + | + | + | + | NI | + | + | + | + | + (osteohyper-trophy) | NI | + | + |
| Periosteal and/or cortical irregular thickening | + | + | NI | + | + | NI | + | + | + | + | NI | + | + | + |
| Erosive lesions | − | NI | NI | + | − | NI | − | + | + | NI | NI | NI | NI | NI |
| Cortical cysts | − | NI | NI | − | + | NI | + | − | − | + | + | + | + | NI |
| Biopsy | NI | NI | NI | Right wrist: fibrous hamartoma | NI | NI | Dorsum of left hand: collagenoma; Left D2: epidermal cyst | NI | NI | Left knee: mild hyperkeratosis, dense sclerotic collagen bundles with absence of skin appendages | NI | NI | NI | D2: hyperkeratosis, diffuse fibrosis |
| Other limb anomalies | − | NI | NI | NI | NI | NI | NI | NI | − | + (anterior bowing of the tibia) | + (AV malformation of ulnar artery, agenesis radial artery) | + (agenesis of radial artery) | NI | NI |
| Mutation identified | + (TSC1) | − (TSC1 and TSC2) | + (TSC2) | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI |
| Fulfills diagnostic criteriaa | Yes | Yes | Yes | Yes | NI | NI | Yes | Yes | Yes | Yes | Yes | Yes | NI | Yesb |
- AV, arteriovenous; D, digit; NI, No information” signifies that there were no clear statements or data (e.g., pictures, X-ray images) in the article regarding the designated feature.
- a Diagnostic criteria according to Northrup and Krueger [2013].
- b Facial angiofibromas plus “lesions characteristic of tuberose sclerosis were found in the brain, the eyes, and the kidneys.”
Our patient is, to our knowledge, the first reported to have clear bilateral involvement (also on physical examination and not only on radiographic assessment). There was a previous report [Lo and Wong, 1993] of a boy with TSC and localized bilateral pachydermodactyly over the proximal phalanges of the left fourth and right middle fingers, but he had only soft tissue swelling, with no bone changes on hand X-ray. These features are distinct from the macrodactyly seen in our patient and some of the others, who had radiographic evidence of bone overgrowth and erosive and/or cystic lesions.
The underlying mechanism for the occurrence of macrodactyly in TSC is not yet completely understood. In the case of our patient, as well as in 10 of the other patients we reviewed, there is periosteal and/or cortical thickening observed on X-rays. In addition, one patient [Sharma et al., 2011] underwent a biopsy of the right wrist, which was compatible with a fibrous hamartoma. These findings are in favor of true tissue hyperplasia, as opposed to lymphedema.
There remains the question of why this occurs. One possible explanation could be the presence of somatic mosaicism for loss of heterozygosity (LOH) at one or more of the TSC loci. Loss of heterozygosity has been previously reported in TSC genes in multifocal micronodular pneumocyte hyperplasia, a rare pulmonary manifestation [Hayashi et al., 2010], as well as renal angiomyolipomas and pulmonary lymphangioleiomyomatosis [Smolarek et al., 1998; Yu et al., 2001; Sato et al., 2002]. The occurrence of localized tissue hyperplasia has also been reported in the case of other neurocutaneous disorders, including neurofibromatosis type 1 [Sabbioni et al., 2010] and Sturge-Weber syndrome [Greene et al., 2009; Babaji et al., 2013]. Neurofibromatosis type 1 is caused by mutations in NF1, a well-known tumor suppressor gene [DeClue et al., 1992]. Recently, it has been demonstrated that Sturge-Weber syndrome is caused by somatic activating mutations in the GNAQ gene, involved in intracellular signal transduction for cell proliferation [Shirley et al., 2013]. Macrodactyly has also been described in association with Klippel-Trenaunay-Weber syndrome [McGrory et al., 1991; Redondo et al., 2009; Ruggieri et al., 2014], which, in turn, has been described concomitantly with TSC in the same individual [Troost et al., 1975]. Klippel-Trenaunay-Weber syndrome is characterized by the presence of localized congenital vascular malformations and disturbed growth of bone or soft tissue [Oduber et al., 2008] and its molecular etiology is still not fully understood, although somatic mosaicism for PIK3CA mutations has been described [Luks et al., 2015]. This findings further support the hypothesis that macrodactyly, as well as other localized tissue hyperplasia, is most likely due to somatic mosaicism for second-hit mutations in individuals with germline mutations in genes related with cell growth and proliferation.
In summary, we present the case of a boy with the clinical and molecular diagnosis of TSC, who also shows bilateral macrodactyly of the first three fingers of both hands, with underlying radiographic changes, namely irregular periosteal thickening of the metacarpal bones and phalanges with global increase in width (more pronounced in the right fingers), associated with diffuse increase of subcutaneous tissue and flexion limitation. The findings in our patient, corroborated by similar findings in other previously described patients, illustrate the fact that macrodactyly in TSC is often associated with deformity and functional limitation, which the clinician must bear in mind, with appropriate referral to specialist care when necessary. The mechanism for this phenomenon has yet to be fully elucidated, although somatic mosaicism for LOH at TSC loci is a plausible explanation.
ACKNOWLEDGMENTS
The authors wish to thank the patient and his family.