Shwachman–Diamond syndrome presenting with early ichthyosis, associated dermal and epidermal intracellular lipid droplets, hypoglycemia, and later distinctive clinical SDS phenotype
Abstract
Shwachman–Diamond syndrome (SDS) is a recessive ribosomopathy, characterized by bone marrow failure and exocrine pancreatic insufficiency (ePI) often associated with neurodevelopmental and skeletal abnormalities. The aim of this report is to describe a SDS patient with early ichthyosis associated with dermal and epidermal intracellular lipid droplets (iLDs), hypoglycemia and later a distinctive clinical SDS phenotype. At 3 months of age, she had ichthyosis, growth retardation, and failure to thrive. She had not cytopenia. Ultrasonography (US) showed pancreatic diffuse high echogenicity. Subsequently fasting hypoketotic hypoglycemia occurred without permanent hepatomegaly or hyperlipidemia. Continuous gavage feeding was followed by clinical improvement including ichthyosis and hypoglycemia. After 14 months of age, she developed persistent neutropenia and ePI consistent with SDS. The ichthyotic skin biopsy, performed at 5 months of age, disclosed iLDs in all epidermal layers, in melanocytes, eccrine sweat glands, Schwann cells and dermal fibroblasts. These iLDs were reminiscent of those described in Dorfman–Chanarin syndrome (DCS) or Wolman's disease. Both LIPA and CGI-58 analysis did not revealed pathogenic mutation. By sequencing SBDS, a compound heterozygous for a previously reported gene mutation (c.258 + 2T>C) and a novel mutation (c.284T>G) were found. Defective SBDS may hypothetically interfere as in DCS, with neutral lipid metabolism and play a role in the SDS phenotype such as ichthyosis with dermal and epidermal iLDs and hypoglycemia. This interference with neutral lipid metabolism must most likely occur in the cytoplasm compartment as in DCS and not in the lysosomal compartment as in Wolman's disease. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Shwachman–Diamond syndrome (SDS: OMIM260400) is a recessive ribosomopathy caused by biallelic loss-of-function mutations [Boocock et al., 2003] in SBDS (OMIM607444). SDS is characterized by bone marrow (BM) failure and exocrine pancreatic insufficiency (ePI), often associated with neurodevelopmental and skeletal abnormalities [Shwachman et al., 1964; Dror et al., 2011]. Very early symptoms or cytopenia at diagnosis are potential signs for severe hematologic complications, malignant or non-malignant [Donadieu et al., 2012]. Rarely, hypoglycemia [Kuijpers et al., 2004; Albrecht et al., 2009] has been reported as a presenting feature of SDS. If ichthyosiform skin lesions have been described in SDS, it is usually not a leading symptom of the disease except in one patient with severe ichthyosis [Goeteyn et al., 1991]. Biochemically, SBDS encodes a highly conserved, broadly expressed 29 kDa ribosome associated protein [Boocock et al., 2006] which functions as an essential cofactor in tightly coupling GTP hydrolysis by elongation factor-like1 (EFL1) on the 60S ribosomal subunit for the release of an accessory protein, the eukaryotic initiation factor 6 (eIF6) allowing the formation of the 80S ribosome for translation initiation [Finch et al., 2011]. By using cell live imaging techniques, it has been shown that patient SBDS protein lacking sumoylation motif in the C-terminal domain has altered nucleocytoplasmic trafficking [Orelio et al., 2011]. The present report describes an SDS patient with early ichthyosis associated with dermal and epidermal intracellular lipid droplets (iLDs), hypoglycemia and later distinctive clinical SDS phenotype.
CLINICAL REPORT
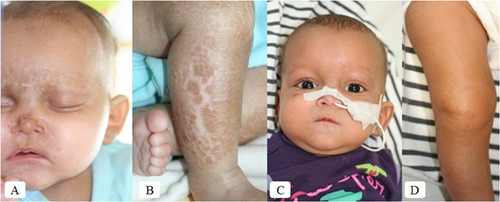
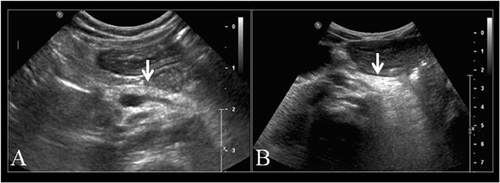
The patient was a girl, born at 354/7 weeks gestational age by cesarean section for fetal distress. Birth weight was 1.540 kg (<10th centile), length 42 cm (<10th centile) and head circumference 28.5 cm (<10th centile). Mother was of Caucasian and father of African origin. At nine days of age, she had a febrile episode with low platelets (Table I) treated with antibiotics, but bacteriological analysis was negative. From birth, she was exclusively fed by human milk. In addition, she daily received elemental iron (15 mg), cholecalciferol (480 UI), and multivitamin supplementation including retinol (1200 UI) and α-tocopherol (0.75 mg). At 3 months of age, she was evaluated for severe failure to thrive. Cardio-pulmonary examination was normal. No hepatosplenomegaly was detected. Neurological evaluation disclosed axial hypotonia. Minor facial dysmorphism including broad nasal bridge, high arched palate and retroverted ears were observed. Her skin was mildly dry. Fine dark scales with hypo-pigmented areas were observed on the median part of her face (Fig. 1A). Larger and thicker dark scales, more typical of lamellar ichthyosiform desquamation, were present on lower limbs (Fig. 1B), lower abdomen and back with areas of mild inflammation. Her hair and eyelashes were scanty and short and the eyebrows nearly absent. Nails were normal. Follicular hyperkeratosis as well xerosis conjunctivae or corneae were not observed. Ophthalmological evaluation was normal including eye fundus. X-rays of the metaphyseal long bones performed at 4 months of age, showed a decrease in mineral bone density, but without metaphyseal chondrodysplasia. An ichthyotic skin biopsy was performed at 5 months of age. At the age of 81/2 months, because of hypoglycemia and poor weight gain (2.700 kg), she was started with continuous nasogastric tube feeding, using special formula (Nutramigen 2®, 100–120 ml/kg, 68 kcal/100 ml), supplemented with human milk. Subsequently, she experienced better weight and length gain as well as ichthyotic skin improvement (Fig. 1C and D) and remained euglycemic. From the age of 14 months, both persistent neutropenia and intermittent thrombocytopenia (Table I) were present, consistent with Pearson or SDS. The patient was started on pancreatic enzyme replacement therapy (Kreon fur Kinder®) and fat-soluble vitamin supplementation was adapted. Pancreatic US, performed at 4 (Fig. 2A) and 10 months of age revealed diffuse high echogenicity. At the age of 16 months, during the day, she had intermittent gavage feeding every 3 hr and during the night, continuous feeding until the age of 3½ years. She was only hospitalized once at the age of 24 months for febrile gastro-enteritis with adenovirus-positive stool sample. At the age of 4½ years, her weight was 9.700 kg (<3rd centile), length 88.6 cm ( 3rd centile), and occipito-frontal circumference 45.7 cm (<3rd centile). She didn't present any more skin lesions, or hypoglycemia. Control pancreatic US performed at 49/12 years of age, showed diffuse high echogenicity. Motor neurological examination was normal with a walking ability reached at the age of 18 months. Her language development was significantly delayed regarding phonological (<−5 SD), syntax (<−2 SD) and memory sentence (−4 SD) abilities and she had low average for lexical aspects with a receptive language level superior to the expressive level (EXAlang 3–6) [Helloin and Thibault, 2006].
| Age (months) | <1 | 3 | 7½ | 9½ | 11 | 12 | 14 | 18 | 25 | 31 | 43 | 57 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | ||||||||||||
| Glucose (3.85–6.38 mmol/L) (d = Dextro) | 5.16 | 1.44 (d) | 3.44 | 3.21 (d) | 3.38 (d) | 3.66 | 4.38 | 4.10 | ||||
| Lactate (0.89–1.69 mmol/L) | 1.84 | 2.53 | 2.22 | 2.18 | 2.18 | 2.2 | 1.4 | 0.62 | ||||
| 3-OH-butyrate (<0.3 mmol/L) | 0 | 0.6 | 0.7 | 0.3 | 0.4 | |||||||
| Free fatty acid (0.2–1.10 mmol/L) (FFA) | 1.53 | 1.86 | 0.24 | 0.60 | ||||||||
| FFA/3-OH-butyrate | 2.56 | 2.62 | 0.82 | 1.50 | ||||||||
| Insulin (2.6–24.9 mIU/L) | 0.2 | 2.0 | ||||||||||
| Aspartate amino transferase (10–35 U/L) | 231 | 149 | 72 | 216 | 72 | |||||||
| Alanine amino transferase (5–40 U/L) | 103 | 66 | 46 | 197 | 66 | |||||||
| Glutamyl transferase (5–70 U/L) | 201 | 168 | 87 | 104 | 83 | |||||||
| CK (120–300 U/L) | 160 | |||||||||||
| Hemoglobin (10.2–13.4 g/dl) | 20.2 | 11.1 | 8.9 | 12.6 | 11.0 | 11.4 | 11.7 | 12.3 | ||||
| Fetal hemoglobin (<1.3%) | 9.7 | |||||||||||
| White blood cells (6.0–17.5 × 109/L) | 7.25 | 7.63 | 11.03 | 11.40 | 64.50 | 39.10 | 31.90 | 4520 | 3490 | |||
| Neutrophils (1.0–8.5 × 109/L) | 2.24 | 2.09 | 3.34 | 0.77 | 0.34 | 0.80 | 0.35 | 0.43 | 0.42 | |||
| Platelets (150–400 × 109/L) | 62 | 345 | 325 | 181 | 118 | 115 | 109 | 140 | 143 | |||
| Total Protein (51–73 g/L) | 50 | 43 | 61 | 61 | ||||||||
| Triglycerides (0.64–2.44 mmol/L) | 0.73 | 0.73 | 0.81 | 0.59 | 0.44 | 0.93 | 0.55 | |||||
| Vitamin A (0.7–1.7 μmol/L) | 0.55 | 1.01 | 1.00 | |||||||||
| Vitamin D 25OH (>75 nmol/L) | 118 | 102 | ||||||||||
| Vitamin E (7.0–21.0 μmol/L) | 7.3 | 14.9 | 21.9 | |||||||||
| Vitamin K1 nmol/L (0.33–1.99) | 1.06 | |||||||||||
| Trypsin (24–79 ng/ml) | <5 | |||||||||||
| Stool (months) | 18 | 38 | ||||||||||
| Fecal fat (1–3 g/24 hr) | 3.4 | 2.7 | ||||||||||
| Pancreatic elastase-1 (>200 μg/g) | <50 |
- Abnormal values are in bold.
Laboratory values between postnatal period and 57 months of age and during hypoglycemic episodes are reported in Table I. Urinary organic acid analyses performed at 4 and 9 months of age did not revealed non ketotic dicarboxylic aciduria. Acyl-carnitine profile performed at 9 ½ months of age on dried blood spot (DBS) during a hypoglycemic episode was not indicative of fatty acid oxidation (FAO) or carnitine cycle defects. Large cytoplasmic lipid vacuoles were not observed in white blood cells.
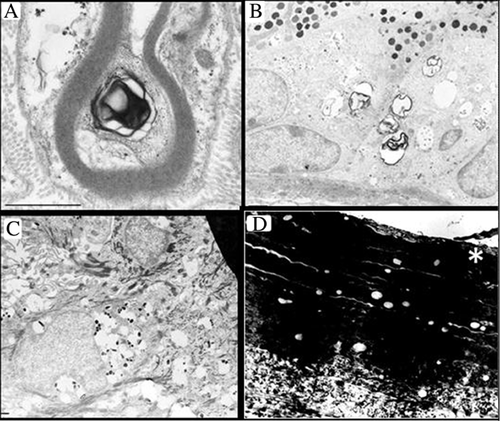
Light microscopy of resin semi-thin sections of the skin biopsy revealed a micro-vacuolization within the epidermis. Numerous small vacuoles were seen in keratinocytes within all epidermal layers, including stratum corneum. The same vacuoles were also observed in melanocytes of the epidermal basal layer, in intradermal fibroblasts and histiocytes and in eccrine sweat glands. By electron microscopy, electron-lucent lipid-like vacuoles measuring 0.5–1 μm and only rarely 2 μm, were mainly found in Schwann cells (Fig. 3A), in clear and dark cells of eccrine sweat glands (Fig. 3B), in melanocytes (Fig. 3C), in epidermal keratinocytes, within keratin layers of stratum corneum (Fig. 3D) and in dermal fibroblasts (not illustrated). Most vacuoles were not membrane-bounded. Few vacuoles contained scattered glycogen granules. Free aggregated β-particles of glycogen were abundant in some eccrine secretory coils (not illustrated). Less specific whorls of pseudomyelinic lamellar structures (Fig. 3A and B) partly wrapped within vacuoles were sometimes found. Dark pigment granules at different stages of maturation appeared inside several vacuoles of melanocytes (Fig. 3C). No abnormal lamellar granules (LGs) were observed in epidermal keratinocytes. These iLDs were reminiscent of those found in Dorfman–Chanarin syndrome (DCS) or Wolman's disease. However, in both LIPA and CGI-58/ABHD5, no pathogenic mutation was found. Finally, by sequencing SBDS, she was found to be compound heterozygous for a previously reported gene mutation (c.258 + 2T>C) arising from gene conversion and resulting in abnormal splicing (Cys84fs3), and a novel mutation (c.284T>G), resulting in an amino acid change (p.Val95Arg), which was inherited from her mother. The father was unavailable for testing.
DISCUSSION
The patient's wide clinical presentation was quite in agreement with the draft consensus guidelines for diagnosis of SDS [Dror et al., 2011]. However, the distinctive clinical features including both hematological characteristics with persistent neutropenia and intermittent thrombocytopenia and ePI, later evolved. Severe growth retardation and biochemical liver abnormalities were supportive findings. Performed at 4 months of age, before full clinical spectrum occurred, the radiographic imaging did not demonstrate the most typical skeletal abnormalities such as metaphyseal dysplasia. Pancreatic diffuse high echogenicity however, detected at 4 months of age, could have raised the suspicion of SDS [Adachi et al., 2005]. Taking into account the age of diagnosis (>3 months) and the presence of cytopenia, this patient belongs to the intermediate risk group for severe cytopenia [Donadieu et al., 2012]. Chronic anemia was not present, but fetal hemoglobin level was increased as reported in 80% of SDS-patients, reflecting stress-hematopoiesis and or ineffective erythropoiesis related to apoptosis [Dror, 2005]. In spite of having persistent neutropenia, recurrent skin or lung infection did not occur. Early ichthyosis however, even if not uncommon in SDS may have been related to nutritional status. At 3 months of age, hypoproteinemia was detected, but it was not directly related to ichthyosis. From birth, she daily received multivitamin supplementation including cholecalciferol, retinol and α-tocopherol. Amongst fat-soluble vitamins, only the vitamin A level was below the normal range when performed later at 18 months of age. Usually, vitamin A deficiency induces squamous metaplasia, especially in glandular epithelium, causing xerophthalmia [Kumar et al., 2003], but not ichthyosis with dermal and epidermal ILDs as in the index patient. Indeed, both light and electron microscopic skin biopsy performed at 5 months of age, revealed variable amounts of iLDs within all epidermal layers which were first reminiscent of the epidermal iLDs described in DCS [Akiyama et al., 2003]. However, in DCS, complete blood count is normal except cytoplasmic lipid inclusions in white blood cells which were not detected in this patient and the abnormal epidermal LGs found in DCS [Akiyama et al., 2003] were not detected in the patient epidermal keratinizing cells. Nevertheless, as described in DCS or other congenital ichthyosis, or in several neurolipidoses, we can speculate for the index patient, that the extra-cellular lamellar membrane structure within the stratum corneum interstices has displayed a limited capacity to incorporate one of the three non-polar lipid species such as free fatty acids (FFA), ceramides or cholesterol, inducing a permeability barrier abnormality leading to a pathophysiology through stimulation of the epidermal hyperplasia which has resulted in hyperkeratosis [Demerjian et al., 2006]. However, DNA analysis did not reveal in this patient any pathogenic mutation in the CGI-58/ABDH5. Secondly, these iLDs were also reminiscent of those observed in Wolman's disease which is a rare autosomal recessive disorder characterized by acid lysosomal lipase deficiency, and hence triglyceride (TG) lysosomal storage. In contrast, in the index patient, Schwann iLDs were not surrounded by a single or double membrane as in Wolman's disease [Byrd and Powers, 1979] and no pathogenic mutation was found in the LIPA. Subsequently she developed persistent neutropenia and ePI which was consistent with SDS. By sequencing SBDS, the index patient was confirmed to be compound heterozygote for a previously reported [Boocock et al., 2003] gene mutation (258 + 2T>C) resulting in abnormal splicing (C84fs3) and a novel mutation (284T>G (p.Val95Arg) inherited from her mother and located in a highly conserved region of the SBDS [Boocock et al., 2003]. The father, although not available, must probably carry the classical c.258 + 2T>C mutation, but the chance of a de novo background is also a possibility [Steele et al., 2014]. As a presenting feature of SDS, hypoglycemia has also been previously reported [Kuijpers et al., 2004; Albrecht et al., 2009]. Nevertheless, the etiology of hypoglycemia remains to be elucidated. In the index patient, plasma insulin level was not suggesting hyperinsulinemia. Both moderate fasting hypoglycemia and hyperlactacidemia were not associated with hyperketosis, hyperlipidemia or permanent hepatomegaly as in glycogen disorders or indicative of gluconeogenesis defects like fructose-1, 6-diphophosphatase deficiency. At 91/2 months of age, the patient's values of β-OH-butyrate (BOB) concentration in relation to blood glucose concentration obtained during fasting hypoglycemia could have discriminated between ketotic and non ketotic hypoglycemia. However, acyl-carnitine profile on DBS was not indicative of FAO or carnitine cycle disorders and urinary organic acid analyses did not detect dicarboxylic aciduria. At 11 and 12 months of age, the rise in FFA concentration during fasting hypoglycemia was not out of proportion with the rise in BOB concentration, being within the 95% predictive value for the relationship between FFA and BOB concentrations [Morris et al., 1996]. Furthermore, serum TG levels were not elevated, even later at the age of 49/12 years, being below the normal range, which could suggest a limited neutral fat availability. A relationship between the dermal and epidermal iLDs and hypoglycemia could only be indirect on the basis of a hypothetically limited neutral fat availability. Indeed the skin, disclosing a very abnormal sign with iLDs, is not primarily adapted like the liver, kidney and intestine to serve as a gluconeogenic organ in order to maintain inter-prandial glucose homeostasis. Intriguingly, some eccrine secretory coil in the index patient disclosed abundant free aggregated β-particles of glycogen suggesting decreased sweating secondary to ichthyosis [Delfino et al., 1991]. Indeed, by metabolizing glucose and glycogen, sweating leads, histologically, to glycogen depletion [Wolfe et al., 1970]. Nevertheless, the clinical gain in both weight and length and the improvement of ichthyotic skins started after the age of 8½ months and followed the nutritional adaptation by using continuous nasogastric special formula feeding (Nutramigen 2®) which contains extensively hydrolyzed protein, fatty acid profile similar to human milk, supplemented with maternal milk. Later, pancreatic enzyme replacement has also contributed to the clinical improvement including fasting both hypoglycemia and ichthyotic skin lesions. An interference of initial nutritional status with the pathophysiology of both ichthyosis and hypoglycemia cannot be excluded. However, following clinical skin lesion improvement, the parent did not accept control skin biopsy. Intra-uterine and postnatal growth retardation, as well as neurodevelopmental delay was also an early feature of the present patient. In an SDS-mouse model, embryos have decreased mass compared to littermate control and SDS brain was apoptotic with hypo-cellularity and neuronal cell death [Tourlakis et al., 2015]. Diffuse echogenicity of the pancreas indicating fatty infiltration was also present in the index patient at the age of 4 months and persisted at the age of 49/12 years of age. The original paper described the pancreas as grossly atrophic and replaced by fatty tissue [Shwachman et al., 1964], which is different from steatosis. In conditional knockout mice, replicating several features observed in human disease, both acinar hypoplasia and marked pancreatic fatty infiltration have been reproduced with loss of zymogen granules [Tourlakis et al., 2012]. This atrophic SDS pancreas phenotype was revealed to be p53- dependent, but has been rescued, including the fatty infiltration, by silencing the p53 [Tourlakis et al., 2015]. Interestingly, mutation-negative SDS patients have normal pancreatic MRI signal intensity despite ePI [Toiviainen-Salo et al., 2008] as for a recently described patient harboring a single SBDS missense mutation and a structural variation in the SBDS locus [Carvalho et al., 2014]. Therefore, in the index patient, defective SBDS protein, as a ribosomopathy could hypothetically interfere with neutral lipid metabolism and play a role in the SDS phenotype including both ichthyosis associated with associated dermal and epidermal iLDs and hypoglycemia. This possible interference with neutral lipid metabolism must most likely occur in the cytoplasm compartment like in DCS and not in the lysosomal compartment as in Wolman's disease.
ACKNOWLEDGMENTS
The authors are most grateful to the parents for their collaboration on this report, to Elodie Gautiez-Silvert and Jean Paul Van Neuwenhuyse for their expertise in echography and to Heather Cattell for revising this manuscript.