Is 1p36 deletion associated with anterior body wall defects?
Abstract
Epispadias and exstrophy of the cloaca, also known as OEIS complex (omphalocele, exstrophy, imperforate anus, spinal defects), respectively constitute the most benign and severe ends of the bladder exstrophy—epispadias complex (BEEC) spectrum. In 2009, El-Hattab et al. reported the first patient with OEIS complex associated with a chromosome 1p36 deletion. Here we report a second patient with 1p36 deletion who also has classic bladder exstrophy, supporting the possible role of genes in this region in the development of BEEC. The absence of omphalocele and imperforate anus in our patient places him toward classic bladder exstrophy while presence of spina bifida and the absence of coccyx suggest an overlap with OEIS complex. An additional differential diagnosis is the pentalogy of Cantrell in our patient as he also has a diaphragmatic hernia and an incomplete sternum. This is the second observation of a ventral midline birth defect in association with 1p36 deletion syndrome, following El-Hattab et al.'s report [2009]. The three genes (NOCL2, DVL1, and MMP23B) discussed as possible candidates are also among the deleted ones in our patient, supporting the possible role of these genes in BEEC spectrum. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
CBE (classic bladder exstrophy) is part of the BEEC (bladder exstrophy—epispadias complex) spectrum [BEEC; OMIM %600057]. CBE includes epispadias, wide pubic symphysis, and anteriorly displaced anus. It is considered as the most common component of the BEEC spectrum [Draaken et al., 2010; Lundin et al., 2010; Zaki et al., 2012]. The term “exstrophy” is the exact description, meaning “turned inside-out.” In males, the urinary tract is exposed from dorsal penis (epispadias) to the level of umbilicus, usually placed inferiorly toward the bladder. The penis is usually short and broad. Rectus abdominis, rectus fascia, and pubic symphysis are separated and the anus may be anteriorly displaced. The upper urinary tract is usually unaffected. However, anomalies such as renal agenesis, horseshoe kidney, and megaureter have been described and reflux occurs in all cases. Indirect inguinal hernias are common due to abnormal abdominal muscle attachment. Bilateral outward rotation of posterior pelvis, retroversion of acetabuli, and progressive diastasis of pubic symphysis result in the outward rotation of lower limbs and so the waddling gait [Sponseller et al., 1995]. Anomalies outside genitourinary system have been seldom reported [Stevenson and Hall, 2006].
Exstrophy of the cloaca, also known as OEIS complex (omphalocele, exstrophy, imperforate anus, spinal defects) belongs to the severe end of the BEEC spectrum [Martinez-Frias et al., 2001; Zaki et al., 2012]. OEIS complex, has been reported in association with several syndromes and chromosomal abnormalities [Keppler-Noreuil, 2001; Martinez-Frias et al., 2001; Hertzberg et al., 2003; Kosaki et al., 2005; Chen, 2007]. El-Hattab et al. [2009] reported the first patient with OEIS complex in association with chromosome 1p36 deletion. Here we report a second patient with 1p36 deletion with bladder exstrophy and overlapping features with OEIS complex supporting the possible role of genes in this region in the development of BEEC.
CLINICAL REPORT
The patient is an Iraqi boy, born as the third live-born child of second-degree cousin parents following an uneventful term twin pregnancy with a birth weight of 1,000 g. His male twin and three sisters are healthy. The twin brother was not present at evaluation. The parents noted they were non-identical. Motor and intellectual developmental milestones were mildly delayed. Bladder exstrophy was detected at birth. He was referred for primary surgical repair of the defect, at the age of 4 years and 6 months.
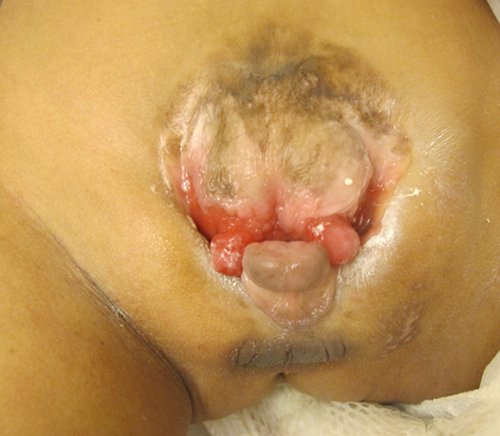
At examination, his height was 88 cm (−3.54 SD), weight was 11 kg (−2.75 SD), and head circumference was 43 cm (−3.9 SD, corrected for height). He had microbrachycephaly, a narrow forehead, flat supraorbital ridges, straight eyebrows, mid-face hypoplasia, broad nasal bridge, bilateral epicanthal folds, a short nose with broad nasal tip, and smooth long philtrum with thick lips and pointed chin. He had mild pectus excavatum deformity more like a midline vertical groove. The lower half of the sternum and xyphoid were non-palpable. Umbilicus was absent. CBE was visible above symphysis pubis, through which urine was dripping. Epispadias, hypoplastic scrotum and absence of palpable testes were noted (Fig. 1). The anus was patent, located anteriorly. Standing up, he had externally rotated legs and a waddling gait.
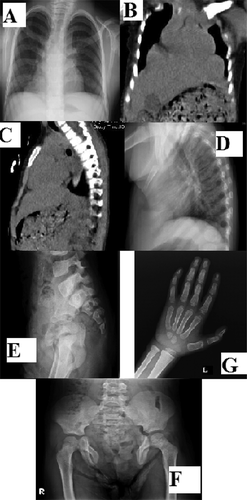
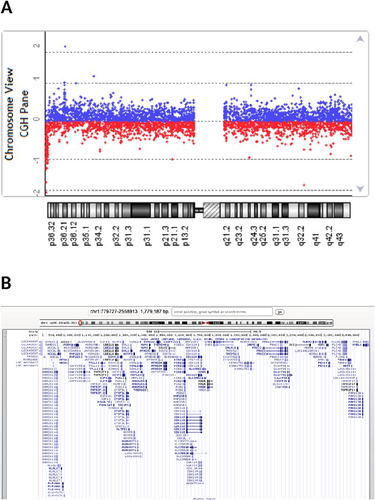
Abdominal ultrasonography revealed normal upper urinary tract development. Chest X-ray showed opacity on lower lung fields and an increased cardiothoracic index (Fig. 2A). CT showed bilateral paracardiac diaphragmatic hernia and the absence of lower sternum (Fig. 2B and C). AP and lateral vertebral radiographs showed absence of xyphoid process and distal two thirds of sternum. Coccygeal elements in the lower spine were also not visible. The pelvis was extremely widened with defective pubic symphysis. AP pelvic radiograph revealed S1 posterior fusion defect due to spina bifida (Fig. 2D–F). Carpal bone age was delayed (Fig. 2G). Chromosomal microarray analysis using Agilent Oligonucleotide 8*60 K showed an apparent 1.77 Mb terminal deletion of chromosome 1p36 (779,727–2,558,913) (Fig. 3A and B).
The patient had immediate surgery after our consultation, the parents were not available during the visit for blood collection and they went back to Iraq. He has not returned for clinical and surgical follow-up. The results were shared with the family via e-mail. They were asked to come back for further genetic investigations i.e., FISH for confirmation of the deletion, parent analysis to demonstrate the de novo character of the deletion. We were unable to receive neither a response nor consent to publish photographs. Nevertheless, the similarities with the patient reported by El-Hattab et al. [2009] encouraged us to report our limited findings.
DISCUSSION
CBE is seen in approximately 1/30,000–1/50,000 Live births. Male to female ratio is 2.3:1 [Shapiro et al., 1984; Martinez-Frias et al., 2001]. Most cases are sporadic, however there are 29 multiplex families reported. Two siblings, two third-degree cousins and two uncle–nephew cases have been described [Reutter et al., 2003]. The risk of recurrence in the same family is said to be less than 1/100 [Boyadjiev et al., 2004]. The risk of recurrence in the offspring of an affected individual is approximately 1/70 Live births, 400-fold higher than average risk [Shapiro et al., 1984]. Twinning seems to be relatively common. So far, 56 twin pairs with CBE have been reported. Concordance rate among monozygotic twins is 5.6-fold higher than dizygotic twins [Reutter et al., 2007]. It is speculated that this entity is multi-factorial, affected by genetic susceptibility and environmental influence. Advanced parental age is also considered as a risk factor for the complex [Boyadjiev et al., 2004]. Insertion/deletion polymorphisms related to ΔNp63 gene have also been reported as a genetic risk factor for BEEC [Wilkins et al., 2012]. Apart from sporadic cases, CBE is listed in association with 14 entities in the London Dysmorphology Database (LMD). Array CGH analysis has demonstrated an association between CBE and 22q11.21, 19p13.12 microduplications, while deletions in 3q and 1q have been reported in patients with OEIS complex [Kosaki et al., 2005; Draaken et al., 2010; Zaki et al., 2012; Draaken et al., 2013].
The embryological pathogenesis for CBE and OEIS complex are intertwined. A large cloacal membrane prevents the mesoderm from growing in, which results in a vulnerable two-layered cloacal membrane that ruptures. The cloacal membrane becomes the roof of the genital folds, rather than the floor. Thus, the musculoskeletal structures are separated, the pelvic girdle is widened, and the urethral groove is on the anterior side. This occurs before the urorectal septation in OEIS complex where the hindgut protrudes between the bladder hemispheres [Stevenson and Hall, 2006].
Monosomy 1p36 is a well delineated microdeletion syndrome with a prevalence of 1 in 5,000 newborns. It is estimated to be responsible for nearly 1% of all “idiopathic intellectual disability” [Giraudeau et al., 2001]. The diagnosis is suggested by distinct facial appearance with straight eyebrows, deep-set eyes, mid-face hypoplasia, broad and flat nasal bridge, long philtrum, pointed chin, and facial hypotonia. Other findings include congenital heart defects (50–75%), eye-vision problems (52%), skeletal anomalies, abnormalities of external genitalia (25%), and renal anomalies (22%). Nearly 50% of individuals with 1p36 deletion are shown to have a “terminal deletion.” The remaining are related to interstitial deletions (30%), complex rearrangements (12%), or derivative chromosomes (7%) [Heilstedt et al., 2003; Battaglia et al., 2008]. We present here, a patient demonstrating microbrachycephaly, mild intellectual disability, and facial features of 1p36 deletion syndrome who was brought into attention due to a congenital ventral abdominal wall defect and CBE. Array CGH analysis demonstrated a 1.77 Mb terminal deletion on 1p36.
In 2009, El-Hattab et al. reported the first patient with OEIS complex, the most severe phenotype in the BEEC spectrum, in association with chromosome 1p36 deletion. The authors reviewed the literature in depth discussing the genetic mechanism of OEIS complex. They concluded that there were no certain candidate genes in the deleted region and the phenotype could be a reflection of a concurrent event. They also mentioned the possibility of a recessive mutation in the 1p36 region which becomes expressed once the intact homologue is deleted.
Here we report a second patient with 1p36 deletion who also has CBE, supporting the possible role of genes in this region in development of BEEC. We were unable to test this hypothesis due to lack of parental DNA in our patient. The absence of omphalocele and imperforate anus in our patient places him toward CBE while the presence of spina bifida and the absence of coccyx, suggest an overlap with OEIS complex. Spinal defects are rare in CBE (6.7%) in contrast to 40–90% prevalence in OEIS complex [Cadeddu et al., 1997; Hertzberg et al., 2003].
The fact that our patient has a discordant twin is an additional interesting overlap with OEIS. It is estimated that 10–30% of patients with OEIS are twin pairs and the prevalence of twin pairs in CBE cases is not as high [Martinez-Frias et al., 2001]. There is one hypothesis suggesting that monozygotic twinning and OEIS have a common defect in early blastogenesis [Lee et al., 1999]. El-Hattab et al. [2009] reviewed the literature for twin pregnancies (26 pairs) in OEIS complex. Concordance rate in monozygotic twins was 50%. Among the 15 discordant twin pairs, 9 were monozygotic, 2 were dizygotic, and 4 were not reported. Our patient is said to be non-identical to his twin brother who has no dysmorphic findings and is developing well. This makes monozygosity less likely and supports the possible role of 1p36 deletion.
Pentalogy of Cantrell also involves anomalies in the ventral midline developmental field. It consists of: (i) a midline supraumbilical abdominal wall defect; (ii) a deficiency in anterior diaphragm; (iii) a defect in lower sternum; (iv) a defect in diaphragmatic pericardium; and (v) congenital cardiac anomalies. It is thought to arise due to a defect in transverse septum of diaphragm, leading to abnormal mesodermal migration, between fourteenth and eighteenth days post-conception [Chen, 2007]. This defect has several pathogenetic mechanisms such as vascular dysplasia, intrauterine mechanical compression, several genetic mutations, and chromosomal abnormalities [Egan et al., 1993; Chen, 2007]. The complete phenotype of the pentalogy is very rare. Several variants involving two or three components have been reported [Denath et al., 1994; Kumar et al., 2008]. Lower sternal defects are associated with nearby organ defects such as the heart, pericardium, diaphragm, and anterior body wall. We therefore believe that the lower sternal defect and bilateral paracardiac diaphragmatic hernia in our patient implies an overlap [Puri and Höllwarth, 2009]. The infraumbilical bladder exstrophy and the coccygeal ossification defect out lie the pentalogy of Cantrell's classical description. Omphalocele and cardiac defects are two most common defects in reported cases of pentalogy of Cantrell. This might suggest a tendency toward reporting patients with more severe and “typical” components of the pentalogy, leading to an underrepresentation of milder phenotypes, as in our patient. Table I summarizes the findings in our patient in comparison to El-Hattab et al.'s paper and the entities in differential diagnoses.
| Classical bladder exstrophy (CBE) | OEIS complex | Pentalogy of Cantrell | El-Hattab et al. [2009] 1p36.33–p36.32 2,4 Mb loss | Our patient 1p36.33–36.32 1,77 Mb loss | |||
|---|---|---|---|---|---|---|---|
| Cardiovascular system | |||||||
| Cardiac anomaly | − | − | + | + | − | ||
| Ectopia cordis | − | − | +/− | − | − | ||
| Diaphragm | |||||||
| Anterior diaphragm deficiency | − | − | +/− | − | − | ||
| Diaphragmatic hernia | − | − | +/− | − | + | ||
| Gastrointestinal system | |||||||
| Omphalocele | − | + | + | + | − | ||
| Imperforate anus | + | − | + | − | |||
| Other | − | +/− | +/− | − | − | ||
| Urinary system | |||||||
| Exstrophy of bladder | + | + | − | + | + | + | |
| Upper urinary tract anomaly | +/− | +/− | − | + | − | ||
| Genital system | |||||||
| Epispadias | +/− | + | − | F | |||
| Scrotal a/hypogenesis | +/− | +/− | − | NA | |||
| Skeletal System | |||||||
| Lower sternal defect | − | − | +/− | − | + | ||
| Sacrococcygeal anomaly | +/− | +/− | − | + | + | + | |
| Vertebral segmentation defect | +/− | + | − | + | + | + | |
| Pelvic anomaly | + | + | − | + | + | + | |
- +, present; −, absent; F, female; NA, not applicable.
- Overlapping findings in the presented patient are demonstrated in divided columns.
In conclusion, we report an additional patient with 1p36 chromosome deletion who also has a ventral midline abdominal wall defect, bladder exstrophy overlapping OEIS complex, and pentalogy of Cantrell. The 1.77 Mb terminal deletion detected in array CGH analysis involves seventy-two genes on chromosome 1p36. The presence of midline abdominal wall defect is noteworthy as this is the second observation of a ventral midline birth defect in association with 1p36 deletion syndrome, following El-Hattab et al.'s report [2009]. The three genes (NOCL2, DVL1, and MMP23B) discussed as possible candidates are also among the deleted ones in our patient, supporting the possible role of these genes in BEEC spectrum [El-Hattab et al., 2009].