Auricular ossification: A newly recognized feature of osteoprotegerin-deficiency juvenile Paget disease
Abstract
We report auricular ossification (AO) affecting the elastic cartilage of the ear as a newly recognized feature of osteoprotegerin (OPG)-deficiency juvenile Paget disease (JPD). AO and auricular calcification refer interchangeably to rigid pinnae, sparing the ear lobe, from various etiologies. JPD is a rare Mendelian disorder characterized by elevated serum alkaline phosphatase activity accompanied by skeletal pain and deformity from rapid bone turnover. Autosomal recessive transmission of loss-of-function mutations within TNFRSF11B encoding OPG accounts for most JPD (JPD1). JPD2 results from heterozygous constitutive activation of TNFRSF11A encoding RANK. Other causes of JPD remain unknown. In 2007, we reported a 60-year-old man with JPD1 who described hardening of his external ears at age 45 years, after 4 years of treatment with bisphosphonates (BPs). Subsequently, we noted rigid pinnae in a 17-year-old boy and 14-year-old girl, yet pliable pinnae in a 12-year-old boy, each with JPD1 and several years of BP treatment. Cranial imaging indicated cortical bone within the pinnae of both teenagers. Radiologic studies of our three JPD patients without mutations in TNFRSF11B showed normal auricles. Review of the JPD literature revealed possible AO in several reports. Two of our JPD1 patients had experienced difficult tracheal intubation, raising concern for mineralization of laryngeal elastic cartilage. Thus, AO is a newly recognized feature of JPD1, possibly exacerbated by BP treatment. Elastic cartilage at other sites in JPD1 might also ossify, and warrants investigation. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Auricular ossification (AO: OMIM 165670), first described by Bochdalek in 1866 upon dissection of a cadaver [Dibartolomeo, 1985], features partial or total rigidity of the external ear (pinnae) sparing the ear lobe [Stites et al., 2003]. Rigid pinnae may result from direct trauma, inflammation, or systemic disease [Dibartolomeo, 1985]. Reports of new etiologies for AO remain infrequent. Auricular inflexibility or radiographic findings are the mainstays for diagnosing AO [Scherrer, 1932; Gossner, 2014]. Multiple other labels for AO include “ectopic ossification of the auricle”, “petrified auricle” and “calcigerous metaplasic ossification”. In some reports, ossification and calcification are used interchangeably [Gordon, 1964; Dibartolomeo, 1985]. Histopathologic studies are few, but differentiate auricular elastic cartilage ossification from calcification [Gordon, 1964; Dibartolomeo, 1985]. Radiographic detection of bony trabeculation in AO has coincided with ossification demonstrated histologically in several publications [Dibartolomeo, 1985; Stites et al., 2003; Machado et al., 2009]. Here, we use AO for either pathologic process.
Ectopic calcification is described as dystrophic or metastatic based on its pathogenesis [Dibartolomeo, 1985]. Dystrophic calcification represents mineral deposition within damaged tissue despite normal circulating levels of calcium and inorganic phosphate (Pi) [Dibartolomeo, 1985]. Trauma and frostbite are the leading explanations, but several metabolic and heritable disorders have also been implicated such as alkaptonuria, acromegaly, Addison's disease, diabetes mellitus, diastrophic dysplasia, familial cold autoinflammatory syndrome 1, hypopituitarism, hypothyroidism, Keutel syndrome, and Primrose syndrome [Dibartolomeo, 1985; Stites et al., 2003; Carvalho and Speck-Martins, 2011]. In contrast, metastatic calcification results from extracellular excesses of calcium and Pi leading to hydroxyapatite (HA) deposition in soft tissues, and may accompany hyperparathyroidism, pseudohypoparathyroidism, tumoral calcinosis, sarcoidosis, milk-alkali syndrome, and vitamin D intoxication [Dibartolomeo, 1985].
Approximately, 160 cases of AO are reported [Stites et al., 2003]. The elastic cartilage components of the external auditory canal are also sometimes calcified [Gossner, 2014]. Several prevalence estimates are available [Dibartolomeo, 1985; Stites et al., 2003; Gossner, 2014]. In 1963, Gordon used radiographs to find a 3% prevalence of AO affecting in-patients with various diagnoses [Gordon, 1964]. In 2014, Gossner identified AO in 19.5% of head CT imaging studies (ages 16–97 years) ordered for various clinical indications [Gossner, 2014]. Gossner's literature review disclosed prevalences of AO varying from 0% (based on palpation of 800 pairs of auricles [Scherrer, 1932]) to 23.5% (based on temporal bone CT [Aw et al., 2011]) [Gossner, 2014].
AO can be painful with applied pressure [Stites et al., 2003]. Additionally, ear pain and hearing loss can occur if the auditory canal is involved [Aw et al., 2011; Buikema and Adams, 2012]. Removal of calcified auricular cartilage benefitted two adults [Lister, 1969; Lari et al., 1989; Stites et al., 2003].
Here, we describe four unrelated people with juvenile Paget disease (JPD: OMIM #239000), three of whom (75%) have AO. JPD is a rare autosomal recessive (AR) disorder featuring extremely rapid bone turnover [Whyte, 2013], elevated serum alkaline phosphatase (ALP) activity (hyperphosphatasemia), along with skeletal pain, fractures, deformity, and hearing loss in childhood from destruction of middle and inner ear bones [Saki et al., 2013; Whyte et al., 2014]. The first report was in 1956 [Bakwin and Eiger, 1956]. They characterized the entity in two Puerto Rican sisters with “fragile bones and macrocranium”. Approximately 60 patients were subsequently reported [Saki et al., 2013]. Without bone antiresorptive treatment, JPD may be fatal during childhood [Whyte et al., 2002]. Notably, JPD patients can have features of pseudoxanthoma elasticum (PXE: OMIM #264800) including acquired diffuse vascular microcalcification leading to retinopathy, blindness, coronary artery occlusion, and perhaps to intracranial aneurysms [Allen et al., 2008; Kerr et al., 2010; Whyte, 2013].
In 2002, a genetic classification of JPD emerged following the discovery of AR transmission of loss-of-function mutations within TNFRSF11B (Tumor Necrosis Factor Receptor Superfamily, member 11B) encoding osteoprotegerin (OPG) in some, but not all, patients with JPD [Cundy et al., 2002; Whyte et al., 2002]. OPG is a receptor member of the tumor necrosis factor (TNF) superfamily, and acts as a decoy for receptor activator of NF-kB ligand (RANKL). Thus, OPG dampens osteoclastogenesis and osteoclast (OC) activity [Whyte et al., 2002; Cundy et al., 2005]. Thereby, loss-of-function mutations in TNFRSF11B in JPD enhance RANK activation, leading to greater OC differentiation, and activity [Whyte et al., 2002]. Phenotype/genotype correlation has been reported for JPD [Cundy, 2002; Whyte et al., 2007; Brunetti et al., 2012; Whyte et al., 2014]. In 2014, when we discovered a unique activating duplication within TNFRSF11A encoding RANK in a girl with JPD, genetic heterogeneity for JPD became apparent [Whyte et al., 2014]. Hence, for JPD we now distinguish JPD1 (OPG deficiency) from JPD2 (RANK activation), while understanding that additional JPD patients have mutations in neither gene, and the etiology of their JPD remains unknown [Whyte et al., 2014].
In 2007, we reported a 60-year-old man with JPD1 who mentioned that his pinnae began hardening at age 45 years [Whyte et al., 2007]. When we recognized AO in two of our three pediatric JPD1 patients, we began this report to document AO as a newly recognized feature of JPD1.
MATERIALS AND METHODS
Case Reports
Informed written consent for all research studies was obtained as approved by the Human Research Protection Office, Washington University School of Medicine, St. Louis, MO.
Patient #1
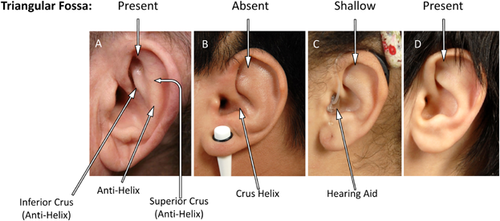
We reported this now 69-year-old Greek man with JPD1 in 2007 [Whyte et al., 2007]. He recalled progressive hardening of his pinnae over several years beginning at age 45 years. There was no pain while sleeping on a pillow, and no skin breakdown. He had become nearly blind from the retinopathy consistent with the microvascular calcification of JPD1 (see below). As expected [Whyte et al., 2013], he was also deaf. Starting at age 32 years, he had received synthetic human calcitonin subcutaneously for 9 years, and then sequentially a series of bisphosphonates (BPs): etidronate at age 40 years for 16 months, pamidronate for 10 years, alendronate for 1.5 years, risedronate for 1–4 months, and most recently denosumab [Polyzos et al., 2014]. He was also treated from age 48 years with 1-α-hydroxyvitamin D3 twice or thrice weekly, and a daily calcium supplement [Whyte et al., 2007]. At age 67 years, nasolaryngeal intubation for a procedure failed, requiring tracheotomy. Years before, an otolaryngologist said his laryngeal anatomy was “atypical.” Recently, at our request, he reported that his friends noted no appreciable change in his voice, and he sent photographs of his external ears, each showing a well-defined antihelix and triangular fossa (Fig. 1A). He reaffirmed that his pinnae hardened beginning at age 45 years.
Patient #2
In 2002, we reported this now 20-year-old Navajo man with JPD1 who presented during infancy and suffered fractures, skeletal deformity, and deafness. We had discovered he was homozygous for selective and complete deletion of TNFRSF11B encoding OPG, thus identifying OPG deficiency as a genetic basis for JPD [Whyte et al., 2002]. He subsequently had nearly lifelong treatment with IV and oral BPs. At age 11 years, unilateral strabismus led to discovery of bilateral carotid artery aneurysms that were treated successfully with occlusion and bypass surgery [Allen et al., 2008]. At age 18 years, retinal angioid streaks were noted. He had been sequentially prescribed salmon calcitonin for 5 years, risedronate for 30 months, and pamidronate for 9 years, and currently receives alendronate. His head circumference was >98th centile. His ears were positioned normally, with small Darwinian tubercles visible, but his prominent antihelix and pinnae were hard and inflexible. The triangular fossae were absent (Fig. 1B). Early photos of patient #2 (not illustrated) show that the triangular fossae were once present.
Patient #3
This 18-year-old American girl of Puerto Rican heritage with JPD1 was referred in 2004 at age 7 years for frequent fractures, expanded “osteoporotic” bones, and hearing impairment diagnosed at age 6 years (subsequently treated with hearing aids). At age 2 years, a femoral diaphysis fractured. JPD was diagnosed based on hyperphosphatasemia, characteristic biochemical and radiographic findings, and positive TNFRSF11B mutation analysis. At age 8 years, we prescribed alendronate twice monthly and ergocalciferol once monthly, and she sustained no further fractures. At age 15 years, she underwent bilateral femoral osteotomies with leg-length correction, at which time endotracheal intubation was reportedly difficult. Concurrently, we noted her pinnae were somewhat rigid. At age 16 years, pinnae hardening was more extensive, causing discomfort while sleeping. Head circumference was >98th centile. Her external ears appeared normal with small Darwinian tubercles, except the pinnae were firm and inflexible, with shallow triangular fossae (Fig. 1C).
Patient #4
This 13-year-old American boy of Greek-Cypriot heritage was referred at age 4 years. He had passed his neonatal hearing test. At age 5 weeks, radiographs of swollen legs revealed periosteal elevation. Non-steroidal anti-inflammatory medication was prescribed for 2 years for presumed Caffey disease. Marked hyperphosphatasemia was discovered at age 4 months. At age 9 months, fracturing began and his limbs progressively deformed. When, at age 13 months, his “Caffey disease” had not resolved, prednisone was given for 2 months. Evaluation of delayed speech at age 21 months revealed an abnormal brainstem audio-evoked response. CT demonstrated no cochleae at age 33 months. Upon referral to us at age 4 years, we diagnosed JPD and pamidronate treatment quickly relieved his skeletal pain. Currently, he is a small boy with a disproportionately large head, but of 52 cm (34th centile). His pinnae were normal and pliable, with well-defined triangular fossae (Fig. 1D).
Medical Literature Review
To determine if AO is a feature of all forms of JPD, we searched the medical literature from 1956 [Bakwin and Eiger, 1956] onward using book chapters and Medline/PUBMED to help identify case reports. We reviewed 23 articles containing 27 case reports having a total of 33 skull radiographs from the 27 published patients (Table SI).
Radiological Review
We also reviewed the radiographs and CT images of our three pediatric patients with JPD1, our pediatric patient with JPD2, and two young women with idiopathic JPD with and without mental retardation.
Mutation Analysis
PCR and DNA sequencing of TNFRSF11B were performed as detailed previously [Whyte et al., 2002; Whyte et al., 2007]. Homozygous mutations of TNFRSF11B were identified in patients #1–4 using VectorNTI® AlignX® software (Invitrogen, Carlsbad, CA).
RESULTS
Medical Literature Review
We (WHM and GSG) examined the 33 published skull radiographs of the 27 JPD patients. Fourteen illustrations were uninterpretable because the skull density was too great to discern the ears. Two patients had external ear densities which appeared consistent with AO (Table SI). Another nine JPD patients had dense auricles, suggesting AO. One image showed no evidence of external ear calcification [Whyte et al., 2002].
Radiographic and Diagnostic Imaging
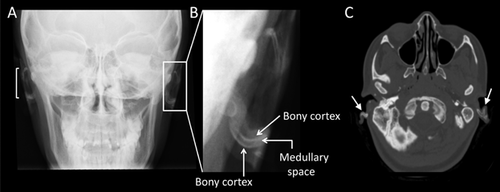
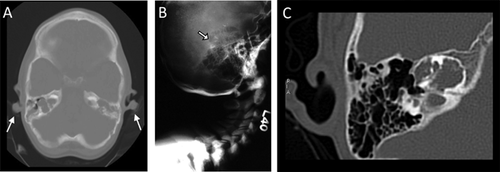
Skull radiographs of two of our four JPD1 patients, patient #2 (Fig. 2A–C) and patient #3 (Fig. 3A–B), revealed evidence of AO, including linear densities indicative of cortical bone surrounding a medullary zone. AO could not be discerned in the skull radiographs of patient #1 (whose skull was extremely dense), nor in patient #4. Additionally, cranial CT of patient #2 (age 10 years, Fig. 2D–E) and patient #3 (age 16 years, Fig. 3C) documented increased radiodensity within the external ears, consistent with ectopic cortical bone outlining medullary spaces within each auricle. In contrast, cranial CT of our younger patient #4 (age 7 years) showed no evidence of AO (Fig. 4A).
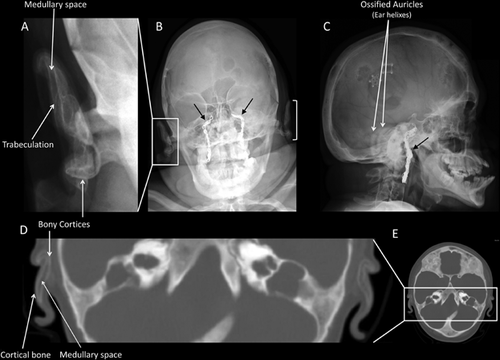
These four patients were not readily available to prospectively evaluate additional sites of elastic cartilage ossification. Therefore, we reviewed previously obtained radiographs and CT images. They showed no clear evidence of calcification of the epiglottis or arytenoids in patients #2, #3, #4. However, these studies were not optimal for interpretation, because only limited views were available (data not shown).
No evidence of AO was seen in the two patients with idiopathic JPD (without OPG or RANK mutations), a 15-year-old student (data not shown), and a 21-year-old woman with mental retardation (Fig. 4B). Similarly, the temporal bone CT of the 11-year-old girl with JPD2 did not reveal AO (Fig. 4C).
Mutation Analysis
All four JPD1 patients carried distinctive homozygous mutations in TNFRSF11B (Table I). The TNFRSF11B mutations for patients #3 and #4 have been reported only in preliminary form (unpublished data).
DISCUSSION
Auricular ossification (AO) was documented radiologically in two of our four patients with JPD1. AO leads to reduced pliability of the external ears, and therefore patient #1 is also likely affected. These findings, plus our review of the JPD literature, indicate that JPD1 is an additional metabolic disorder that causes AO.
The presence of AO in JPD1 adds to concerns about ectopic calcification and ossification in the OPG-deficient state. In 1971, Mitsudo reported the autopsy of an 18-year-old man with JPD and skin findings of pseudoxanthoma elasticum [Mitsudo, 1971]. Microcalcification was found in his coronary arteries and renal cortex arterioles. However, no mention of AO was made [Mitsudo, 1971]. In 1998, Bucay et al. [Bucay et al., 1998] described the first murine model of Opg deficiency, and unexpectedly found ectopic calcification of the aorta and renal arteries, two sites where OPG is expressed. These investigators offered that OPG could be called “osteovasculoprotegerin” [Bucay et al., 1998]. Furthermore, in 2006, Kanzaki et al. [Kanzaki et al., 2006] reported auditory ossicle resorption in 6–15 week-old Opg-/- mice, describing Opg as an “audioprotegerin”. However, these investigators made no mention of AO despite placing their electrodes for auditory brain-stem response measurements adjacent to the pinnae [Kanzaki et al., 2006]. Circulating levels of OPG are elevated in people with atherosclerosis, and while detrimental roles for OPG have been hypothesized, recent evidence from rodent models seems to instead support a protective role for OPG [Jono et al., 2002; Reid and Holen, 2009; Van Campenhout and Golledge, 2009]. Reportedly, murine OPG can promote endothelial cell survival, but whether elevation of OPG in human atherosclerotic disease is a compensatory response, decreasing plaque calcification, or plays an active role in the disease process, is unanswered [Reid and Holen, 2009].
We recognize that OPG deficiency in JPD1 ultimately increases circulating bone ALP (BAP) activity [Whyte et al., 2002]. BAP in the skeleton controls extracellular levels of inorganic pyrophosphate (PPi), an inhibitor of calcification, by acting as a cell-surface phosphohydrolase of PPi [Ronchetti et al., 2013]. Thus, chronic elevation of BAP in JPD1 could diminish PPi levels and engender vascular calcification and promote auricular calcification [Dibartolomeo, 1985]. Of interest, adults with classic Paget disease of bone, who are also typically hyperphosphatasemic, seem at risk for ectopic calcification including angioid streaks from retinopathy [Gass and Clarkson, 1973]. In fact, decreased biosynthesis of PPi underlies generalized arterial calcification of infancy (GACI) (OMIM #208000), an AR disorder featuring microcalcification (HA deposition) within elastic fibers of arterial blood vessels [Rutsch et al., 2003]. GACI is caused by loss-of-function mutations within the ectonucleotide pyrophosphatase/phosphodiesterase1 gene (ENPP1) and more rarely within ATP-binding cassette subfamily C number 6 (ABCC6) which encodes a transmembrane transport protein (ABCC6) with unknown physiologic substrates [Rutsch et al., 2003; Nitschke et al., 2012]. ENPP1 hydrolyzes extracellular nucleotide triphosphates to liberate PPi, and thus ENPP1 deficiency reduces extracellular PPi levels leading to ectopic calcification [Otero et al., 2013]. Inhibition of ectopic mineralization in GACI is achieved using BPs that are essentially synthetic analogues of PPi [Fleisch, 1995], including the first-generation BP, etidronate, and more recently amino-BPs.
Lomashvili et al. in 2014 [Lomashvili et al., 2014] transplanted normal aortic tissue into a murine model of GACI and found calcium deposited in the graft, implicating low extracellular PPi levels in the formation of ectopic calcification. Sheen et al. [2015], described a murine model of medial vascular calcification that selectively overexpresses ALP only in the vascular smooth muscle cells. They suggested local depletion of PPi and an alteration in the extracellular milieu resulted in calcium phosphate nanocrystal deposition. This mineral deposition occurred prior to osteogenic transdifferentiation of vascular smooth muscle [Sheen et al., 2015], and thus suggested the calcification preceded the ossification. These examples implicate reduced levels of PPi as an important factor in the generation of ectopic calcification.
The precise pathogenesis of AO in the pinnae is unclear, and may begin with excesses of bone morphogenetic proteins (BMPs), which transform elastic cartilage connective tissue cells to having osteogenic potential [Dibartolomeo, 1985; Nakamura et al., 2003; Buikema and Adams, 2012]. Transdifferentiation of aortic valve interstitial cells (myofibroblasts), in response to active inflammation and lipid infiltration, engenders localized calcific nodules [Mohler et al., 2001]. This process has been implicated in triggering heterotopic bone formation in 13% of calcified heart valves. There is no evidence in JPD1 that auricular calcification precedes AO. Nonetheless, perhaps in JPD1 the elevated ALP activity from high bone turnover leads to low extracellular levels of PPi and triggers calcification of elastic cartilage with subsequent activation of osteogenic precursors (as hypothesized for the calcification of human aortic valves [Mohler et al., 2001]) leading to the development of ossification in the pinnae. However, if this were the simple explanation, one might find AO in all types of JPD.
Several other disorders (see Introduction section) are associated with AO. Defects in Gsα (GNAS) cause Albright hereditary osteodystrophy (AHO) associated with subcutaneous calcification and progressive osseous heteroplasia with osteoblast differentiation leading to intramembranous ossification. The ectopic ossification may be conditioned by modifier genes that alter Gsα expression from the unaltered allele, another component of the G protein GTPase signaling pathway, or other pathways regulating osteoblast differentiation [Weinstein et al., 2013]. In alkaptonuric ochronosis from homogentisic oxidase deficiency (HGD), excess homogentisic acid is enzymatically oxidized forming polymeric deposits in cartilage. Stiff discolored pinnae and subcutaneous nodules occur [Sanji et al., 2011]. The subcutaneous tissues contain cartilage with hyalinized collagen and the calcification appears secondarily and late in the process [Sanji et al., 2011]. Diastrophic dysplasia from altered SLC26A2 features characteristic cystic ear swelling in 2/3 of infants with classic disease [Bonafé et al., 2013]. Sulfation of cartilage matrix proteoglycans is markedly diminished, leading to defective extracellular matrix and cartilage. Defects in long bone, tracheal, laryngeal, and peribronchial cartilage have been noted [Bonafé et al., 2013]. Proteoglycan undersulfation in the cartilaginous matrix leads to the cystic changes in auricular cartilage and cystic swelling of the pinnae without preceding trauma. However, the mechanism remains unclear. Calcification and ossification of the pinnae are late findings, typically noted in adulthood [Cushing et al., 2011]. Keutel syndrome includes inner ear deafness and abnormal cartilage ossification or calcification caused by mutation within the matrix Gla protein (MGP) gene. Cartilage ossification is the main presenting feature. Extracellular MGP inhibits calcification. Auricular cartilage ossifies without tissue reaction or prior calcification [Khosroshahi et al., 2014]. Lastly, Primrose syndrome has characteristic calcification of the pinnae [Carvalho and Speck-Martins, 2011]. Here, missense mutations in zinc finger- and BTB domain-containing protein 20 (ZBTB20) are suspected of having a dominant-negative effect altering glucose metabolism, neurogenesis, muscle development, and post-natal growth [Cordeddu et al., 2014]. There may be dysregulation of genes controlling calcium homeostasis leading to the intracranial and external ear calcifications. Thus, these heritable disorders of bone development and mineralization demonstrate the complex varied interplay between multiple pathways that promote or inhibit mineral deposition [De Vilder and Vanakker, 2015]. Elucidation of the pathogenesis of ectopic mineralization in each disorder will be especially informative concerning the normal balance among promoters and inhibitors of mineral deposition and ossification.
Interestingly, all four of our JPD1 patients had significant exposure to BPs. Whether the BPs promoted their AO by stabilizing HA crystals, or conversely protected them against further AO by decreasing endogenous ALP levels and increasing PPi levels is unclear. Patient #1, with a relatively mild TNFRSF11B mutation [Whyte et al., 2007] appeared by clinical history to have developed AO 5 years after starting BP treatment. Following uneventful treatment with etidronate that perhaps was protective, he noted ear hardening after the third of 10 years of pamidronate therapy. His apparently late-onset AO after prolonged BP treatment could implicate BPs or his treatment with 1-α-hydroxyvitamin D3 and supplemental calcium.
Our literature review of JPD revealed no mention of AO. However, the radiographs contained in 23 articles regarding 27 patients yielded two individuals who likely had AO, and nine others perhaps with AO. Because several reports represented the pre-BP era; that is, prior to 1971 [Fleisch, 1995], it seems AO may occur naturally in JPD (i.e., without exposure to BPs). Thus, OPG may directly or indirectly prevent not only calcification of the vasculature, but also ossification of elastic tissue. A minority of JPD patients lack TNFRSF11B mutations and therefore have no OPG deficiency [Whyte et al., 2014]. In the patient with JPD2 [Whyte et al., 2014], as well as in our two enigmatic JPD patients without OPG or RANK defects [Whyte et al., 2002, 2014], we found no evidence of AO. However, the JPD2 patient was only 13 years-old when reported in 2014, and had received pamidronate infusions (the last seven cycles essentially at yearly intervals). Her young age and relatively low exposure to BP therapy confound any speculation about her lack of AO.
Elastic cartilage is present in the external ear, but also found in the epiglottis and arytenoids [Dibartolomeo, 1985; Sato et al., 1990]. Therefore, we searched for evidence of arytenoid calcification or ossification in our patients’ radiologic studies all of which were obtained for other indications. In fact, a concern for airway cartilage calcification in JPD1 arose because patients #1 and #3 were reported by anesthesiologists to be difficult to intubate, and patient #1 required tracheotomy. The few relevant images showed no conclusive evidence of ectopic calcification. Nevertheless, radiographs of the airway prior to intubation and general anesthesia may be prudent for JPD patients. CT of the airway would provide more detail if elastic cartilage calcification or ossification was found. The clinical implications of airway ossification could be more significant than AO.
ACKNOWLEDGMENTS
Mr. Vinieth Bijanki assisted with figure design and data management. Mrs. Sharon McKenzie helped prepare the manuscript.