Multigenerational autosomal dominant inheritance of 5p chromosomal deletions
Abstract
Deletion of the short arm of chromosome 5 (5p-) is associated with phenotypic features including a cat-like cry in infancy, dysmorphic facial features, microcephaly, and intellectual disability, and when encompassing a minimal critical region, may be defined as Cri-du-Chat syndrome (CdCS). Most 5p deletions are de novo in origin, and familial cases are often associated with translocation and inversion. Herein, we report three multigenerational families carrying 5p terminal deletions of different size transmitted in an autosomal dominant manner causing variable clinical findings. Terminal 5p deletions and the mode of inheritance were clinically characterized and molecularly analyzed by a combination of microarray and fluorescence in situ hybridization analyses. Shared phenotypic features documented in this cohort included neuropsychiatric findings, poor growth, and dysmorphic facial features. This study supports newly recognized effects of aberrant SEMA5A and CTNND2 dosage on severity of autistic and cognitive phenotypes. Comparative analysis of the breakpoints narrows the critical region for the cat-like cry down to an interval less than 1 Mb encompassing a candidate gene ICE1, which regulates small nuclear RNA transcription. This study also indicates that familial terminal 5p deletion is a rare presentation displaying intra- and inter-familial phenotypic variability, the latter of which may be attributed to size and gene content of the deletion. The observed intra-familial phenotypic heterogeneity suggests that additional modifying elements including genetic and environmental factors may have an impact on the clinical manifestations observed in 5p deletion carriers, and in time, further high resolution studies of 5p deletion breakpoints will continue to aid in defining genotype–phenotype correlations. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
5p deletion syndrome (5p-) is a genetic disorder resulting from a terminal or interstitial deletion of the short arm of chromosome 5, and is among the most common deletion syndromes. When encompassing a minimal critical region, monosomy 5p may be characterized as Cri du Chat syndrome (CdCS). The incidence of CdCS ranges from approximately 1:15,000 to 1:50,000 in live-born infants, and about 1:350 in the intellectually disabled population [Cerruti Mainardi, 2006]. The hallmark features of CdCS include a high-pitched monotonous cry, low birth weight, microcephaly, hypotonia, poor growth, and developmental delay [Mainardi et al., 2001]. Specific craniofacial findings include round face, bilateral epicanthal folds, hypertelorism with a broad nasal bridge, down slanting palpebral fissures and short philtrum [Cerruti Mainardi, 2006]. CdCS is also associated with variable intellectual disability and neuropsychiatric manifestations including autistic behaviors [Church et al., 1995; Moss et al., 2008]. In addition, some individuals with CdCS may present with retinal vessel abnormalities, optic atrophy, or congenital renal and heart defects. Most patients with CdCS, however, do not have other major congenital malformations [Cerruti Mainardi, 2006].
Individuals with 5p deletion demonstrate variability in the clinical presentation. The high degree of phenotypic variability is mainly attributed to the size of the deletion, which can range from 5 to 40 Mb, and the gene content of the monosomic region [Simmons et al., 1998]. A more severe phenotype and cognitive impairment were reported to be associated with larger deletions [Wilkins et al., 1983; Cornish et al., 1999]. A number of genotype–phenotype correlation studies have been performed allowing for definition of critical regions associated with specific manifestations, such as the region for speech delay at band 5p15.3 [Church et al., 1995], the region for cat-like cry between proximal 5p15.3 and distal 5p15.2 [Overhauser et al., 1994; Gersh et al., 1995; Levy et al., 2002; Wu et al., 2005], and the region associated with dysmorphic features at band 5p15.2 [Overhauser et al., 1994; Church et al., 1995; Gersh et al., 1995; Church et al., 1997; Mainardi et al., 2001]. Multiple chromosomal locations have been implicated in intellectual disability and microcephaly, including 5p15.31 [Rossi et al., 2005], 5p15.2, 5p15.1, and 5p13p14 [Overhauser et al., 1994; Gersh et al., 1995; Mainardi et al., 2001]. Several genes on 5p, including SEMA5A (Semaphorin 5A), CTNND2 (delta-catenin), and TERT (human telomerase reverse transcriptase), have been studied in association with autism spectrum disorder, intellectual disability, schizophrenia, or altered fetal development [Medina et al., 2000; Zhang et al., 2003; Harvard et al., 2005; Vrijenhoek et al., 2008; Weiss et al., 2009].
Approximately, 90% of 5p deletions are de novo in origin, and ∼10% of cases arise from translocation events [Niebuhr, 1978; Cerruti Mainardi, 2006]. Autosomal dominant inheritance of 5p deletion has been rarely documented [Kushnick et al., 1984; Church et al., 1995; Cornish et al., 1999; Fang et al., 2008]. Here we report three multi-generational kindreds with 5p deletions, demonstrating intra- and inter-familial phenotypic heterogeneity. Each family carries a relatively small terminal deletion with breakpoints distal to 5p15.1. Phenotypic variability is manifested among the affected members of each kindred, and suggests that modifying factors contribute to the clinical presentation. On the basis of the breakpoints delineated in this study, along with correlation from the literature, our data further support the refinement of the critical region for the cat-like cry phenotype.
METHODS
Informed consent was obtained from study participants.
Cytogenomic Microarray Analysis (CMA)
Cytogenomic microarray analysis was performed using the Affymetrix Genome-Wide Human SNP 6.0 array (Affymetrix Inc., Santa Clara, CA), which contains about 1.8 million genetic markers/probes. More than half the probes were non-polymorphic for the detection of copy number variation (CNV), with the remainder providing SNP calls for genotype and loss of heterozygosity analysis. DNA was isolated from peripheral blood lymphocytes using the QIAamp DNA Blood Mini Kit (Qiagen Inc., Valencia, CA). Arrays were prepared in accordance with the manufacturer's instructions with an input amount of 500 ng of genomic DNA. Data were analyzed using Genotyping Console v4.0 (Affymetrix Inc. Santa Clara, CA) with an in silico reference library established at Washington University School of Medicine, and visualized by the Affymetrix Chromosome Analysis Suite v2.0. The genomic linear positions are given relative to GRCh37/hg19.
Fluorescence In Situ Hybridization Analysis (FISH)
Peripheral blood samples were cultured using standard cytogenetic methods for 72 hr with phytohemagglutinin (PHA) stimulation. Fluorescence in situ hybridization was performed with standard techniques using the Vysis LSI EGR1/D5S23, D5S721 dual color probe (Abbott Laboratories, Des Plaines, IL) for EGR1 at 5q31.2 and the locus of D5S23/D5S271 at 5p15.2, and the mixed TelVysion probe for subtelomeric regions of chromosome 5: C84c11/T3 at 5pter and D5S2907 at 5qter (Abbott Laboratories, Des Plaines, IL).
CLINICAL REPORTS
Family I
The proband (III-1 in Fig. 1A), was a 5 year 9 month old male with developmental delay, and autism spectrum disorder. His birth weight was 4.08 kg (∼95th centile), length 54.6 cm (>95th centile), and occipitofrontal circumference (OFC) 36.83 cm (∼98th centile). He had tracheomalacia, gastroesophageal reflux, and an unusual cat-like cry during the newborn period. His family reported problems of constipation. There was concern for developmental delay prior to the age of 3 years, as developmental milestones included rolling over and sitting unsupported at 9 months, and walking at 2 years with an uncoordinated gait. He had self-stimulatory and self-injurious behaviors, and had recently been diagnosed with autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD). He was attending school in a mainstream setting with a special education teacher. He was receiving physical, occupational, and sensory integration therapies. Previous testing for Fragile X syndrome was normal. At his initial clinic visit, his weight was in the 25th centile for age, height 16th, and OFC between 50th and 75th. He was difficult to examine due to anxiety. He had myopia and wore corrective lenses. He had a broad forehead, deep-set eyes, and preauricular pit on the left, but no other unusual findings.
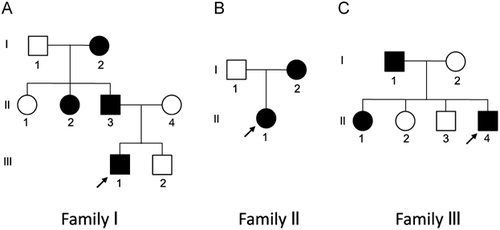
The father of the proband (II-3) had a medical history that included a cat-like cry during infancy, a ventricular septal defect (VSD), pharyngeal flap surgery, and constipation. He had no hearing problems. He experienced speech articulation problems and learning difficulties, particularly in mathematics, spelling, and reading, requiring special services in school. However, he was able to obtain a bachelor's degree in computer science and maintains a full time job in a related field. The mother of this proband (II-4) was healthy, but had a history of learning problems, anxiety, and attention deficit disorder (ADD). The younger brother of the proband (III-2) was developmentally normal and did not have the cat-like cry in infancy.
A paternal aunt (II-2) demonstrated a cat-like cry in infancy. She was born at 37 weeks with a birth weight of 2.34 kg (10–25th centile) and length 45.7 cm (10th centile). She had no eyebrows or fingernails at birth. She was diagnosed with hypothyroidism and a small esophagus that required surgical repair. She had no known hearing problems. She had myopia and wore glasses. She was diagnosed with speech articulation problems, learning difficulties, and hyperactivity in elementary school and required resource room help. She had a pharyngeal flap procedure in the 7th grade, which improved her articulation problems. She graduated from high school.
The paternal grandmother of the proband (I-2) was 157.5 cm tall. She has worn glasses since age of 10. She experienced multiple ear infections as a child, but had no known hearing issues. She was diagnosed with hypothyroidism, had a heart condition described as a “skip beat,” and had high cholesterol. She had premature graying of hair. She had anxiety issues. Her overall development history was not available. She graduated from high school, but had learning problems.
Family II
The proband (II-1 in Fig. 1B) was a three-day-old female infant referred for genetic evaluation due to her dysmorphic craniofacial features and a weak, high-pitched cry. She was born at 38-2/7 weeks of gestation to a 28 year G1P1 mother. The pregnancy was complicated by a history of maternal developmental disabilities and autism, as well as depression treated with Celexa and Zoloft. Her weight was 3.67 kg (75–90th centile), length was 53.5 cm (75–90th centile), and head circumference was 32.5 cm (10th centile). She had relative microcephaly. Her anterior fontanelle was flat and soft. She had large bilateral epicanthal folds with a broad flat nasal bridge and flattened nasal tip. She had large flat ears with uplifted earlobes. She also had a small skin tag on her left cheek that was less than a millimeter in size. She had micrognathia and increased nuchal skin folds with neck webbing both anteriorly and posteriorly. Her neck appeared short. She had a large patent ductus arteriosus which eventually closed spontaneously, desaturations with feedings, and an oxygen requirement. She had a normal brain MRI scan, upper GI study and abdominal ultrasound. By the time of discharge from the NICU, she was taking formula by mouth, but required thickened feeds due to dysphagia and aspiration.
She was seen again at 13 months of age. By that time, the feeding difficulties had improved. Her weight was 7.8 kg (far below the 1st centile), height was 72.5 cm (21st centile) and head circumference 41.9 cm (less than the 1st centile). However, when these parameters were plotted on the Cri du Chat growth chart, she was approximately at the 50th centile for all parameters. New physical findings included a bifid uvula and intermittent esotropia. She was rolling over but was still unable to get into sitting unassisted and was not able to pull to a stand. She was grasping and transferring objects and was cooing but not babbling.
At 2 ½ years of age, her weight was 10.6 kg (5th-10th centile), length was 82.2 cm (5–10th centile) and head circumference was 45 cm (less than 5th centile). She was crawling on her hands and knees. She could stand up and take steps with someone holding her hand or supporting her under her arms. She could say several words and use one sign. She could clap, wave, and finger feed herself.
The mother of the proband (I-2) has a history of autism, depression, developmental disabilities, and scoliosis. The father of the proband (I-1) also has autism and some developmental concerns.
Family III
The proband (II-4 in Fig. 1C) was a 3-year-old Caucasian male. He was first seen at 4 days of life in the hospital for a history of poor feeding, bradycardia and a hoarse cry due to transient bilateral vocal cord dysfunction. He was born at 39 weeks gestation to a 29-year-old G4P4 mother. Apgars were 7 at 1 min and 8 at 5 min. Upon admission to the NICU, his weight was 2,630 gm (3rd centile), length 45 cm (less than the 3rd centile), and OFC 32 cm (below 3rd centile). He was hospitalized at 2 ½ years of age for croup and stridor. He has otherwise been healthy. His echocardiography and brain MRI have been unremarkable.
Developmentally, he walked at approximately 22 months of age. At 3 years of age, he could express about 10 words and could make simple 2 and 3 word sentences. The patient was able to run and occasionally can climb stairs by himself, but is uncoordinated. He received different therapeutic services for his developmental delay. At 3 years 11 months of age, his weight was 11.6 kg (1st centile), length 91 cm (10–20th centile), and OFC 45.7 cm (1st centile). On physical exam, he was found to have epicanthal folds, upslanting palpebral fissures, periorbital puffiness, infraorbital creases, micrognathia, short philtrum, and simple auricles. There was bilateral fifth finger clinodactyly.
While the father of the proband (I-1) did not display dysmorphic features, he was in special education due to some learning difficulties and behavioral issues. The proband's 9-year-old sister (II-1) is reported to have learning difficulties and ADHD. At 9 years, 9 months of age, her length was at the 55th centile, weight at the 70th centile, and OFC at 10–25th centile. She has had no dysmorphic features. Another sister of the proband (II-2, 8 years old) was diagnosed with bipolar disorder and ADHD. She is in special education. The proband's brother (II-3, 6 years old) had unilateral vocal cord paralysis at birth and the family reports a small hole in the heart. He is also reported to have behavioral issues. The mother has no health or cognitive problems.
RESULTS
Family I
Microarray analysis using the Affymetrix SNP 6.0 platform revealed the proband (III-1 in Fig. 1A) carries an approximately 10.36 Mb deletion encompassing the distal portion of 5p (arr[hg19] 5p15.33p15.2(17,630-10,381,437)x1), which overlaps the region associated with CdCS (Fig. 2A and Fig. 3). The breakpoint of the deletion was estimated to be located between basepair positions 10,381,437 and 10,382,584 of chromosome 5 based on build 37 of the human genome sequence (hg19), within the first intron of MARCH6. FISH analysis using the LSI EGR1/D5S23, D5S721 probe confirmed the deletion in the proband (Fig. 2B) and revealed that it was paternally inherited. On the basis of the inheritance pattern, further FISH studies were conducted on at-risk family members, and revealed that the 5p deletion was also present in the proband's mildly affected paternal grandmother and moderately affected paternal aunt (Fig. 1A; FISH data not shown).
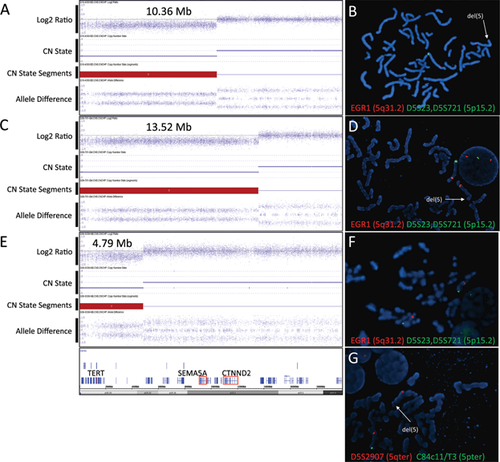
Family II
Microarray analysis revealed the proband (II-1; Fig. 1B) harbors an approximately 13.52 Mb terminal deletion encompassing the distal portion of 5p (arr[hg19] 5p15.33p15.2(17,630-13,532,695)x1), which overlaps the region associated with CdCS (Fig. 2C and Fig. 3). The breakpoint of the deletion was estimated to be located between basepair positions 13,532,695 and 13,536,409 of chromosome 5 based on build 37 of the human genome sequences (hg19). FISH analysis confirmed the deletion in the proband (Fig. 2D) and detected the deletion in her mother (data not shown) (Fig. 1B).
Family III
Microarray analysis revealed the proband (II-4; Fig. 1C) harbors an approximately 4.79Mb deletion encompassing the distal portion of 5p (arr[hg19] 5p15.33p15.32(17,630-4,805,799)x1) (Fig. 2E). The breakpoint of the deletion was estimated to be located between basepair positions 4,805,799 and 4,811,846 of chromosome 5 based on build 37 of the human genome sequences (hg19) and occurred distal to the cat-like cry critical region (Fig. 3). FISH analysis of at-risk family members demonstrated the deletion is distal to the D5S23/D5S721 locus, though detectable using a subtelomeric 5p probe (Fig. 2F, G), and present in father I-1 and sibling II-1, but not in II-2 and II-3 indicating that the deletion in the proband is of paternal origin.
DISCUSSION
Deletions involving the short arm of chromosome 5 demonstrate variable phenotypic effect. The size and gene content of the deletion are the main determinants of the phenotypic variability associated with 5p deletion. Genotype–phenotype correlation studies have identified the critical regions for CdCS-associated clinical features, including the cat-like cry (5p15.3), speech delay (5p15.3), as well as facial dysmorphism, microcephaly and severe intellectual disability (5p15.2) [Cerruti Mainardi, 2006]. In general, patients who have larger deletions with break points in the proximal portion of 5p14 and 5p13 tend to have more significant cognitive impairment. Those who have smaller deletions with break points in band 5p15.3 exhibit less significant problems.
Most individuals with CdCS have a de novo deletion. However, familial translocations and other rearrangements have also been reported [Mainardi et al., 2001]. The multigenerational inheritance of a 5p deletion has been documented only in a few kindreds [Kushnick et al., 1984; Church et al., 1995; Cornish et al., 1999; Fang et al., 2008]. In this report, we present three familial cases of 5p deletion syndrome characterized by molecular and/or cytogenetic methodology. These terminal deletions were inherited in an autosomal dominant manner and manifest variable intra-familial phenotypes.
Monosomy for a contiguous stretch of genes at the distal portion of chromosome 5p is associated with CdCS. Several genes in the region including SEMA5A, CTNND2, and TERT are of particular interest because their products play major roles during embryonic and neuronal development [Simmons et al., 1998; Medina et al., 2000; Zhang et al., 2003]. The SEMA5A gene encodes a membrane protein with a semaphorin domain and several thrombospondin type-1 repeats, and acts as a signaling molecule that regulates axonal guidance and neuronal migration during development. Haploinsufficiency of SEMA5A may contribute to the neurodevelopmental phenotype observed in CdCS [Simmons et al., 1998], as recent evidence suggests, this gene may play a role in autism susceptibility [Weiss et al., 2009]. The CTNND2 gene is expressed early during neuronal development, and encodes an adherens protein known to control neuronal cell motility. Monosomy of the CTNND2 gene has been demonstrated in association with severe intellectual disability and developmental delay in CdCS [Medina et al., 2000].
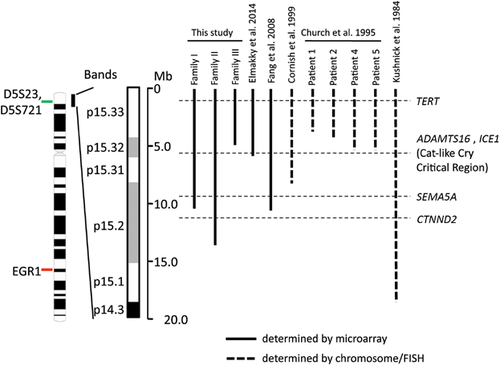
Among the three families presented here, the copy number of SEMA5A and CTNND2 was variable, due to deletion size and location. Family II, harboring the largest 5p deletion among the kindreds at 13.52 Mb, demonstrated monosomy for both the SEMA5A and CTNND2 genes. In contrast, in family I, the 10.36 Mb loss results in monosomy for SEMA5A and disomy for CTNND2 as the deletion breakpoint occurs between the two genes. Finally, the relatively small 4.79 Mb deletion in Family III does not encompass either gene, resulting in a disomic state for SEMA5A and CTNND2 (Fig. 3). The presence of CTNND2 in a two-copy state may explain the milder cognitive impairment observed in Families I and III as compared to individuals who harbor larger 5p deletions. The literature supports this observation on the basis of historic CdCS studies, as well as more recent observations, in that duplication of 5′ CTNND2 and deletion of the 3′ portion of the gene in a CdCS patient is hypothesized to result in amelioration of the cognitive phenotype [Sardina et al., 2014]. Small exonic deletions of CTNND2 have also been reported in individuals with low normal IQ and learning problems with or without autistic features or developmental delay [Asadollahi et al., 2014]. In addition, a recent study identified that deleterious variants in the CYFIP1, DLG1, PLXNA3, and CTNND2 genes are enriched in severely affected patients in female-enriched multiplex families (FEMFs) with severe autism and demonstrated the loss-of-function effect of CTNND2 in autism and neurodevelopment by in vivo and in vitro functional analyses [Turner et al., 2015].
Interestingly, PLXNA3, with enriched variants in FEMFs, encodes a receptor for SEMA5A [Turner et al., 2015], which is located distal to CTNND2 (Fig. 3). The SEMA5A locus has been identified to be among candidate genomic regions associated with autism risk by several Genome-wide association studies (GWAS) [Weiss et al., 2009; Prandini et al., 2012]. SEMA5A expression is significantly decreased in the brain of individuals with autism [Melin et al., 2006]. Moreover, SEMA5A expression quantitative trait loci significantly overlap rare autism-specific CNVs and known autism candidate genes, suggesting that SEMA5A acts as a common downstream effector for known autism CNVs and genes [Cheng et al., 2013]. The presence of autistic behaviors in Families I and II with monosomy of SEMA5A, while not in Family III with normal copy number of the gene, provides additional evidence to support the hypothesis that SEMA5A is an autism susceptibility locus and that aberrant modulation of SEMA5A expression plays an important role in pathophysiology of autism.
The telomerase reverse transcriptase (TERT) gene is localized at 5p15.33 and encodes the rate-limiting component of telomerase complex essential for telomere length maintenance and sustained cell proliferation [Zhang et al., 2003]. It has been demonstrated that individuals with CdCS who are haploinsufficient for TERT have shorter telomere length compared to age-matched unaffected individuals [Zhang et al., 2003]. Insufficient or suboptimal expression due to monosomy of TERT in our three familial cases is likely to play a contributory role in associated clinical phenotypes. Consistent with this, the affected paternal grandmother (I-2; Fig. 1A) in Family I manifested premature graying of hair, which has been reported in other individuals affected by CdCS [Kushnick et al., 1984].
Notably, these three genes account for only a proportion of the phenotype observed in 5p deletion syndrome, and concomitant loss of other genes in the region is certain to play an important role in the clinical features as well. Broader study of gene function and structure in the 5p region is necessary to further elucidate genotype–phenotype correlations. Intriguingly, on the basis of the breakpoints defined in this study, relative to phenotype, our data further support the narrowing of the cat-like cry critical region. The proband in Family III has dysmorphic features, developmental delay, microcephaly, and poor growth, but does not exhibit a cat-like cry. Therefore, the microdeletion in this family does not appear to include the critical region for the cat-like cry in CdCS [Wu et al., 2005]. Recently, Elmakky et al. [2014] reported a small terminal deletion (arr[hg19] 5p15.33p15.32(22,178-5,539,182)x1) due to an unbalanced 5;15 chromosomal translocation causing atypical CdCS phenotypes including cat-like cry [Elmakky et al., 2014]. On the basis of the breakpoints from Family III in our study, along with the proband reported by Elmakky, we narrow down the critical region for cat-like cry to a 733.4 Kb region at 5p15.32 (4,805,799–5,539,182) encompassing only three genes, LINC01020 (a long noncoding RNA gene), ADAMTS16, and ICE1. The developmental role of these two protein-coding genes remains largely unknown, and both are potential candidates for the cat-like cry phenotype associated with CdCS. ADAMTS16 belongs to the ADAMTS (a disintegrin and metalloproteinase domain with thrombospondin motifs) gene family, encoding secreted proteinases. The ICE1 product is one protein component of little elongator complex ELL, which regulates the transcription of small nuclear RNAs (snRNA) [Smith et al., 2011]. Interestingly, a zebrafish ICE1 mutant (ice1hi3655dTg) demonstrated abnormal pharyngeal arches (Phenotype Annotation (1994–2006) (2006) Mutant Data Curated from Older Literature. ZFIN Historical Data). It is notable that pharyngeal anomalies, which could contribute to high-pitch voice, are frequently observed in patients diagnosed with Cri-du Chat. Since ICE1 regulates transcription and may be more susceptible to haploinsufficiency, the potential involvement of ICE1 in mammalian larynx formation warrants further investigation.
We identified seven reports of familial 5p terminal deletions in the literature (summarized in Fig. 3 and Table I). Among them, only one was characterized by high-resolution microarray technology with a deletion of 10.5 Mb [Fang et al., 2008]. The breakpoint was estimated to be located within the ropporin 1-like gene (ROPN1L). This deletion is similar to that identified in Family I of our study. In the Fang et al. study, the affected members showed variable clinical features. Two affected females had moderate intellectual disability, as well as psychoses (auditory hallucinations, paranoia, delusional thinking, and self- laughing), rarely associated with CdCS and not present in the other three affected male family members nor in Family I of this study. The remaining cases reported in the literature were characterized by conventional chromosome and FISH analysis and therefore the size and breakpoints could not be precisely determined [Church et al., 1995; Cornish et al., 1999; Kushnick et al., 1984]. The breakpoints were estimated to be located within band 5p15.3 for five cases, and band 5p15.1 for the case reported by Kushnick et al., 1984 (Table I and Fig. 3). All affected members of the family reported by Cornish et al. [1999] presented high-pitched cat-like cry, but variable learning disability, psychomotor development, and intelligence quotient (IQ) ranging from 70 to 105. Four cases from Church et al. [1995] are atypical without cat-like cry and facial dysmorphism, and only presented mild phenotypes with developmental delay and low-normal intelligence with a variable expressivity among affected family members. The 5p deletion with the breakpoint within 5p15.1 in the family of Kushnick et al. [1984] is the largest among all familial cases summarized in Table I. Both the proband and the mother had cat-like cry in infancy. The proband exhibited hypotonia and several facial dysmorphism. The mother had mild intellectual disability, several dysmorphic features, and premature graying of hair.
| Reference | Age/Sex | 5p deletion | High-pitched, cat-like voice | DD | ID | Neuropsychiatric behavior | Speech | Morphological facial features | Other |
|---|---|---|---|---|---|---|---|---|---|
| Fang et al. [2008]: | |||||||||
| Proband: I-1 | 62 y/F | 5p15.2-pter (∼10.5 Mb) | + | ND | Moderate I.D. | Uncontrolled temper, self-injuries, and aggressiveness, auditory hallucinations, delusion of persecution, self-talking, self-laughing | ND | − | |
| Daughter: II-1 | 41 y/F | + | No high-pitched voice | ND | Moderate I.D. | Hot temper and aggressive behavior, self-talking, self-laughing, delusions of persecution, auditory hallucinations | ND | − | |
| Son: II-2 | 39 y/M | + | ND | ND | Mild I.D. | − | ND | − | |
| Son: II-3 | 36 y/M | + | ND | ND | Mild I.D.: FIQ = 60 PIQ = 63 VIQ = 57 | − | ND | − | |
| Grandson: III-1 | 15 y/M | + | ND | ND | Moderate I.D.: FIQ = 47 PIQ = 46 VIQ = 44 | − | ND | − | |
| Cornish et al. [1999]: | |||||||||
| Father | 39.5 y/M | 5p15.3-pter | + | − | Mild ID: FIQ = 95; PIQ = 100 VIQ = 93 | NA | Weak verbal skills | No-mild facial dysmorphism | Right-handed |
| Offspring1 | 13.9 y/ND | + | + | ND | FIQ = 101; PIQ = 92; VIQ = 86 | Normal psychomotor development | Weak expressive language | Slight facial dysmorphism in infancy | Right-handed, attended mainstream school, normal reading skills |
| Offspring2 | 10.8 y/ND | + | + | Failure to thrive | Mild ID: FIQ = 70; PIQ = 75; VIQ = 70 | Mild psychomotor development | Same as offspring1 | Same as offspring1 | Right-handed, attended a school for children with mild-moderate learning disability, poor reading skills |
| Offspring3 | 6.7 y/ND | + | + | Failure to thrive | FIQ = 105; PIQ = 113; VIQ = 99 | Mild psychomotor development | Same as offspring1 | Same as offspring1 | Right-handed, attended mainstream school, poor reading skills |
| Church et al. [1995]: | |||||||||
| Proband 1: III-1 | ND | 5p15.3-pter | − | ND | Mild ID | ND | SD | − | Profound hearing loss, microcephaly, preauricle skin tags |
| Mother: II-1 | ND | + | − | ND | Low-normal intelligence | ND | ND | − | Epilepsy |
| Maternal grandfather: I-1 | ND | + | − | ND | Low-normal intelligence | ND | ND | − | |
| Proband 2: VI-1 | ND | 5p15.3-pter | raspy voice; no catlike cry | − | − | ND | SD | − | |
| Mother: III-1 | ND | + | raspy voice | + | Mild ID | ND | SD | − | |
| Maternal uncle: III-10 | ND | + | raspy voice | + | Mild ID | ND | SD | − | |
| Proband 4: III-5 | ND | 5p15.3-pter | − | + | − | ND | SD | − | |
| Brother: III-6 | ND | + | − | + | − | ND | SD | − | |
| Paternal cousin: III-3 | ND | + | − | + | − | ND | SD | − | |
| Mother: II-3 | ND | + | − | − | − | ND | SD | − | |
| Paternal aunt: II-2 | ND | + | − | − | − | ND | SD | − | |
| Paternal grandmother: I-2 | ND | + | − | − | − | ND | SD | − | |
| Proband 5:II-1 | ND | 5p15.3-pter | − | Mild DD | Low-normal intelligence | ND | Mild SD | ND | No typical CdCS features |
| Mother:I-1 | ND | + | ND | ND | Low-normal intelligence | ND | ND | ND | |
| Kushnick et al. [1984]: | |||||||||
| Proband | Newborn | 5p15.1-pter | + | ND | ND | ND | ND | Outward, downward slant to the eyes; broad based nasal bridge; smooth philtrum; prominent, protruding, and slightly posteriorly rotated ears; high arched palate | Hypotonia; no skeletal dysmorphisms |
| Mother | 21 y/F | + | + | ND | Mild ID: IQ = 65 | ND | ND | Ocular hypertelorism, pre-auricular tag, and premature hair graying | Required special training program |
- DD, developmental delay; SD, speech delay; ID, intellectual disability; IQ, intelligence quotient; FIQ, full IQ; PIQ, performance IQ; VIQ, verbal IQ; ND, no data.
| Age/Sex | 5p deletion | High-pitched, cat-like voice | Critical genes deleted | DD | ID | Neuropsychiatric behavior | Speech | Morphological facial features | Other | |
|---|---|---|---|---|---|---|---|---|---|---|
| Family I: | ||||||||||
| Proband III-1 | 5.75 y/M | 5p15.2-pter (∼10.36 Mb) | + | TERT, ADAMTS16, ICE1, SEMA5A | +, growth delay | IQ = 84 | Autism, ADHD, anxiety | Poor | Deep-set eyes, preauricular pit on the left | Constipation, tracheomalacia and reflux, uncoordinated motor skills |
| Father II-3 | ND/M | + | + | ND | Mild learning difficulties | ND | Poor, articulation difficulties | ND | Constipation, short-sighted, had pharyngeal flap procedure, VSD | |
| Paternal aunt II-2 | ND/F | + | + | ND | Mild learning difficulties | ND | Poor, articulation difficulties | No eyebrows at birth | Hypothyroidism, myopia, small esophagus, had pharyngeal flap procedure | |
| Paternal Grandmother I-2 | ND/F | + | ND | ND | Mild learning difficulties | Anxiety | ND | ND | Hypothyroidism, short sighted, premature hair graying | |
| Family II: | ||||||||||
| Proband II-1 | 3 d/F | 5p15.2-pter (∼13.52Mb) | + | TERT, ADAMTS16, ICE1, SEMA5A, CTNND2 | +, growth delay | Mild to moderate IDb | ND | ND | Microcephaly, retrognathia, broad nasal bridge, flattened nasal tip, dysplastic ears, large bilateral epicanthal folds, facial skin tag, short webbed neck | Widened nuchal fold, hypotonia, patent ductus arteriosus |
| Mother I-2 | ND/F | + | ND | ND | Mild learning difficulties | Autism, depression | ND | ND | Scoliosis | |
| Family III: | ||||||||||
| Proband II-4 | 3 y/M | 5p15.32-pter (4.79Mb) | −a | TERT | +, growth delay | ND | ND | Delay | Microcephaly, epicanthal folds, upslanting eyes, micrognathia, small philtrum, intraorbital creases, simple auricles | Very thin hair, mild strabismus, bilateral 5th finger clinodactyly, overriding toes (2 over 3) |
| Father I-1 | ND/M | + | ND | ND | Mild ID with learning difficulties | Some behavioral issues | ND | No dysmorphic facial features | ||
| Sister II-1 | 9 y/F | + | ND | ND | Mild ID with significant learning difficulties | ADHD | ND | No dysmorphic facial features | ND | |
- DD, developmental delay; ID, intellectual disability; ADHD, attention deficiency and hyperactivity disorder; IQ, intelligence quotient; ND, no data.
- a Transient hoarse voice due to bilateral vocal cord dysfunction.
- b Assessed in 2011.
5p deletion syndrome is a well-described clinical entity, but multigenerational inheritance has been rarely documented. We present a detailed clinical and cytogenetic/molecular characterization of three kindreds with familial 5p deletions. Intra-familial phenotypic variability suggests that additional modifying elements including genetic and environmental factors may impact the clinical manifestations observed in patients with 5p deletions. In this line of thought, it is notable that in families I and II neuropsychiatric and developmental concerns were documented in both the maternal and paternal parent of the proband. It is also of interest that within each multigenerational family, the proband was an affected child diagnosed in the newborn or early childhood period. This occurrence may be associated with a more severe presentation in early development, and/or may be attributed to improved access to healthcare and increased knowledge among healthcare providers allowing for early referral and diagnosis of this genetic disorder. In addition, the results from these clinical data further support refinement of the critical region for cat-like cry to a ∼700 Kb region at 5p15.32 encompassing only two coding genes, ADAMTS16 and ICE1. Over time, the use of high-resolution technology such as SNP arrays and next-generation sequencing techniques to define breakpoints at basepair resolution will continue to aid in the delineation of genotype–phenotype correlations in 5p deletion syndrome.
ACKNOWLEDGMENTS
We thank the families and patients for their participation in this study.




