Genetic characteristics, clinical spectrum, and incidence of neonatal diabetes in the Emirate of AbuDhabi, United Arab Emirates
Abstract
Neonatal diabetes mellitus (NDM) can be transient (TNDM) or permanent (PNDM). Data on NDM from the Gulf region are limited to few studies on PNDM.The objective of this study was to describe the genetic and clinical spectrum of NDM and estimate its incidence in AbuDhabi, capital of the United Arab Emirate (UAE). Patients were identified from the pediatric diabetes clinics and sequencing of known NDM genes was conducted in all families. Twenty-five patients were identified. Incidence during 1985–2013 was 1:29,241 Live births. Twenty-three out of twenty-five had PNDM (incidence 1:31,900) and 2/25 had TNDM (incidence 1:350,903). Eleven out of twenty-five had extra-pancreatic features and three had pancreatic aplasia. The genetic cause was detected in 21/25 (84%). Of the PNDM patients, nine had recessive EIF2AK3 mutations, six had homozygous INS mutations, two with deletion of the PTF1A enhancer, one was heterozygous for KCNJ11 mutation, one harboured a novel ABCC8 variant, and 4/21 without mutations in all known PNDM genes. One TNDM patient had a 6q24 methylation defect and another was homozygous for the INS c-331C>G mutation. This mutation also caused permanent diabetes with variable age of onset from birth to 18 years. The parents of a child with Wolcott–Rallison syndrome had a healthy girl following pre-implantation genetic diagnosis. The child with KCNJ11 mutation was successfully switched from insulin to oral sulphonylurea. The incidence of PNDM in Abu Dhabi is among the highest in the world and its spectrum is different from Europe and USA. In our cohort, genetic testing has significant implications for the clinical management. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Neonatal diabetes mellitus (NDM) is defined as hyperglycemia diagnosed in the first 6 months of life and needing treatment. NDM can be permanent (PNDM) or transient (TNDM). Using the current definition, the incidence of NDM was 1:89,000 in Germany [Grulich-Henn, 2010], 1:90,000 in Italy [Iafusco, 2012], and 1:160,949 in Austria [Wiedemann, 2010]. In these studies, the proportion of TNDM patients was 10%, 40%, and 70% respectively. The remaining epidemiological data on NDM were confined to PNDM [Bappal, 1999; Slingerland, 2009; Habeb, 2012a; Kanakatti, 2013; Abujbara, 2014; Demirbilek, 2015].
NDM has a monogenic etiology with mutations in more than 20 genes identified in over 80% of patients [Rubio-Cabezas, 2013; Flanagan, 2014]. These mutations cause diabetes through different mechanisms resulting in variable phenotypes. In PNDM the genotype and phenotype is largely influenced by paternal consanguinity. Heterozygous mutations in the KATP channel genes (KCNJ11 and ABCC8), are the commonest cause of PNDM in outbred populations [Vaxillaire, 2004; Suzuki, 2007; Edghill, 2008; Støy J, 2008; Rubio-Cabezas, 2013]. Most patients with these mutations have isolated diabetes and achieve better glycemic control when treated with oral sulphonylurea (SU) rather than insulin [Pearson, 2006; Rafi, 2008]. However in consanguineous families, PNDM is more likely to be syndromic with recessive EIF2AK3 mutations being the most frequent cause [Docherty, 2013; Rubio-Cabezas, 2013]. Approximately 70% of TNDM subjects have imprinting defects of chromosome 6q24 and the remaining cases are caused by mutations in KCNJ11, ABCC8, INS, or HNF1B. Patients with 6q24 TNDM typically present in the first week of life, go into remission at a mean age of 12 weeks and more than 40% have extra-pancreatic congenital anomalies [Docherty, 2013; Rubio-Cabezas, 2013]. Interestingly some 6q24 TNDM patients relapse during adolescence [Flanagan, 2014].
In the Gulf region, data on NDM are limited to two studies on PNDM: In Oman, an incidence of 1:45,000 was reported; but the authors used a cut-off age of 4 weeks to define PNDM and no patient had genetic testing [Bappal, 1999]. However a recent study, from the highly consanguineous population of northwest Saudi Arabia, reported a high PNDM incidence as high as 1:21,000 with most patients having PNDM as a part of a rare familial autosomal-recessive syndrome [Habeb, 2012b].
We aimed to describe the genotype and phenotype of patients with NDM diagnosed in Emirate of Abu Dhabi, United Arab Emirates (UAE), and estimates its incidence during 1985–2013.
MATERIALS AND METHODS
The study was conducted according to the Helsinki Declaration and a signed written consent for genetic testing was obtained from all families.
Emirate of Abu Dhabi is the largest of seven Emirates forming the UAE. Its 2.2 million populations is a mixture of Arabs and expats from neighboring Asian countries and most of them are split between the city of Abu Dhabi, which is the Capital of UAE, and Al-Ain city. The birth rate in the Emirate of AbuDhabi is among the highest in the world and consanguinity is highly practiced [Tadmouri, 2009]. The pediatric diabetes service is provided by a number of units in AbuDhabi and Al-Ain through a compulsory health insurance system regulated by Health Authority Abu Dhabi (HAAD).
Patient Identification
Patients with diabetes diagnosed before 6 months of life were identified from the main pediatric diabetes clinics in Abu Dhabi and Al-Ain cities. NDM patients referred for genetic testing to the Exeter molecular genetic laboratory from Emirate of Abu Dhabi were also included in the study. PNDM was defined as continuous insulin requirement for at least 6 months and patients who underwent spontaneous remission with or without relapse were considered to have TNDM. The incidence of NDM was calculated based on the total number of live births during the study period as supplied by the HAAD (http://www.haad.ae/HAAD/LinkClick, accessed June 2015). Detailed demography, phenotype, genotype, and clinical management were collected from the charts. A child with PNDM and pancreatic aplasia born in 2015 and three patients with diabetes due to mutations in genes known to cause NDM but diagnosed after the age of 6 months were described; but these four patients were excluded from the calculation of NDM incidence during 1985–2013.
Genetic Testing
Genetic testing was performed in the Exeter Molecular Genetics Laboratory, UK. DNA was extracted from peripheral blood using the standard methods and direct sequencing of KCNJ11, ABCC8, INS, and EIF2AK3 was initially conducted in all patients according to previously described methods [Edghill, 2008]. In patient 11.1, loss of methylation at 6q24 was detected using methylation-specific PCR as described by Mackay et al. [2008]. Patients with no identifiable mutations were subsequently analyzed for all known NDM genes using a targeted next generation assay [Ellard, 2013].
RESULTS
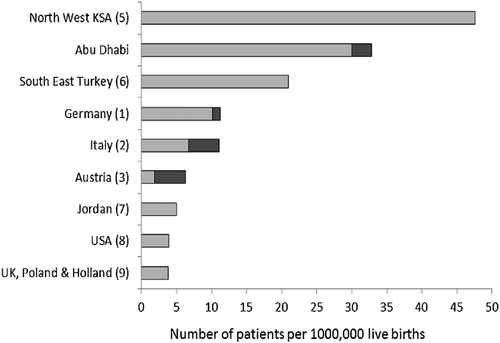
During 1985–2015 25 patients (16 males) from 15 families with NDM were identified by 4 units. Five patients were diagnosed in Al-Ain and the rest were from Abu Dhabi. All 25 patients were pancreatic autoantibody-negative, two (8%) had TNDM and 23 (92%) were PNDM. Based on the life births of 701,807 during 1985–2013, the incidence of NDM in Emirate of AbuDhabi during this period was at least 1 in 29,241 live births, with a rate of 1:31,900 for PNDM and 1:350,903 for TNDM. A comparison of this incidence to rates in other populations is illustrated in Figure 1.
Genotype
The genetic causes identified in the whole cohort are shown in Table I.
| Family patient | Year of diagnosis | Consanguinity | Gender | B.weight and Gestation | Age at diagnosis | Mutation | Phenotype | Prognosis/ current management | |
|---|---|---|---|---|---|---|---|---|---|
| 1.1 [Kanakatti, 2013] | 2007 | Yes | Male | 2.0 kg, Term | 6 weeks | EIF2AK3 p.W430X (c.1290G>A) | WRS: PNDM, Liver failure, anemia | Died at 5 years | |
| 1.2 [Kanakatti, 2013] | 2011 | Yes | Female | 1.9 kg, Term | 7 weeks | EIF2AK3 p.W430X (c.1290G>A) | WRS: PNDM, Liver failure, anemia, neutropenia, SD | 2 years old; MDI insulin 0.85 U/kg/day | |
| 1.3 [Kanakatti, 2013] | 2007 | Yes | Male | 2.3 kg, Term | 6 weeks | EIF2AK3 p.W430X (c.1290G>A) | WRS: PNDM, Liver failure, anemia, neutropenia, SD | Died at 4 years | |
| 1.4 [Kanakatti, 2013] | 2011 | Yes | Female | 2.4 kg, Term | 10 weeks | EIF2AK3 p.W430X (c.1290G>A) | WRS: PNDM, Liver failure, anemia, neutropenia, SD | 4 years old; MDI insulin 0.9 U/kg/day | |
| 1.5 [Kanakatti, 2013] | 2003 | Yes | Male | 1.9 kg, Term | 7 weeks | EIF2AK3 p.W430X (c.1290G>A) | WRS: PNDM | Died at 7 years | |
| Liver failure, anemia, neutropenia, SD | |||||||||
| 1.6 [Kanakatti, 2013] | 2013 | Yes | Female | 2.4 kg, Term | 6 weeks | EIF2AK3 p.W430X (c.1290G>A) | WRS: PNDM, Liver failure, anemia, neutropenia, SD | Died at 18 months | |
| 2.1 [Kanakatti, 2013] | 2012 | Yes | Male | 2.4 kg, Term | 14 months | EIF2AK3 | WRS: diabetes, Liver failure, pancytopenia, SD | 8 years old; had liver transplantation at 2 years; on insulin MDI | |
| p.I650T (c.1949T>C) | |||||||||
| 3.1 [Kanakatti, 2013] | 1997 | Yes | Male | 2.1 kg | 8 weeks | EIF2AK3 p.G956E (c.2867G>A) | WRS: PNDM, hepatitis | Died at 15 years | |
| Term | anemia, SD | ||||||||
| 4.1 [Kanakatti, 2013] | 2009 | Yes | Male | 1.6 kg | 8 weeks | EIF2AK3 | WRS: PNDM, Recurrent hepatitis | 6 years; Insulin pump therapy | |
| Term | p.E524X (c.1567_1570del) | 0.8 U/kg/day | |||||||
| 5.1 [Kanakatti, 2013] | 2012 | Yes | Female | 1.9 kg | 6 weeks | EIF2AK3 p.? (c.1427-?_2490+?del) | WRS: PNDM, Liver failure | Died at 18 months | |
| Term | |||||||||
| 6.1 [Mackay et al., 2008] | 1993 | Yes | Male | 1.9 kg | 6 weeks | INS c-331C>G | Isolated PNDM | 22 years; MDI insulin | |
| Term | 1.1 U/kg/day | ||||||||
| 6.2 [Mackay et al., 2008] | 1990 | Yes | Male | 2.4 kg | 18 years | INS c-331C>G | Isolated diabetes | 25 years; insulin 0.8 U/kg/day | |
| Term | |||||||||
| 6.3 [Mackay et al., 2008] | 1987 | Yes | Female | 2.3 kg | 13 years | INS c-331C>G | Isolated diabetes | 28 years; Insulin pump therapy 0.7 U/kg/day | |
| 6.4 [Mackay et al., 2008] | 1985 | Yes | Female | 2.1 kg | 6 weeks | INS c-331C>G | Isolated PNDM | 30 years; insulin MDI 1.2 U/kg/day | |
| 6.5 | 2013 | Yes | Male | 2.1 kg | 1 hr | INS c-331C>G | Isolated PNDM | 1 year; insulin MDI | |
| 7.1 | 2009 | Yes | Female | 1.4 kg | Day 2 | INS c-331C>G | Isolated TNDM | 6 years; Off insulin | |
| 7.2 | 2010 | Yes | Female | 1.3 kg | Day 2 | INS c.331C>G) | Isolated PNDM | 5 years; MDI insulin | |
| 7.3 | 2013 | Yes | Male | 1.145 kg | Day 2 | INS c.331C>G | Isolated PNDM | 20 months; MDI insulin | |
| 7.4 | 2013 | Yes | Male | 800 g; 31 weeks | Day 2 | Died before mutation testing | Isolated NDM | Died at 2 weeks of sepsis | |
| 8.1 | 2012 | Yes | Female | 2.4 kgTerm | 6 weeks | KCNJ11(p.R201C, c.601C>T) | Isolated PNDM | 2 years; switched to SU dose at 9 month | |
| 9.1 | 2000 | No | Male | 2.4 kgTerm | 25 weeks | Heterozygoup.H410Y ABCC8 variant | Isolated PNDM | 15 years; Insulin pump therapy 1.3 U/kg/day | |
| 10.1 | 2006 | Yes | Male | 1.9 kgTerm | 6 weeks | 6p24 (loss of maternal methylation locus) | TNDM, macroglossia, flat occiput, single simian crease, hypertelorism, spindle shaped fingers, multiple pigmented macules over the trunk. nystagmus, agenesis of corpus callosum, absent septum pelucidum | 8 years; Off insulin | |
| 11.1 | 2015 | Yes | Male | 2.4, term | 2 weeks | deletion of the PTF1A enhancer | PNDM; Abscent pancreas on MRI scan | 4 months, MDI insulin, 0.9 U/Kg/day | |
| 11.2 | 2006 | Yes | Male | 2.1, term | 3 months | deletion of the PTF1A enhancer | PNDM; Abscent pancreas on MRI scan | 11 years, insulin pump 0.8 U/Kg/day | |
| 12.1 | 1999 | No | Male | 2.3 kgTerm | 13 weeks | No mutation detected* | PNDM; Abscent pancreas on CT scan | 16 years; Insulin pump therapy 0.9 U/kg/day | |
| 13.1 | 2001 | No | Female | 2.1 kg; Term | 4 months | No mutation detected* | Isolated PNDM | 15 years; MDI insulin1.0 U/kg/day | |
| 14.1 | 2004 | Yes | Male | 1.8 kg; 30 weeks | 5 months | No mutation detected* | Isolated PNDM | 11 years; MDI insulin 0.8 U/kg/day | |
| 15.1 | 2005 | Yes | Male | 1.9 kg; Term | 4 months | No mutation detected* | PNDM; Down's syndrome | 10 years; MDI insulin 0.9 U/kg/day | |
- WRS, Wolcott-Rallison Syndrome; PNDM, Permanent neonatal diabetes mellitus; TNDM, Transient Neonatal diabetes; SD, Skeletal dysplasia; MDI, multiple daily injections.
- * Patients were tested for 20 genes known to cause PNDM.
The genetic defects were identified in 21/25 (84%). Homozygous EIF2AK3 mutations were the commonest cause of PNDM being identified in 9/23 patients from five families, followed by recessive INS c-331C>G mutation detected in 7/23 patients from two unrelated families. Deletion of the PTF1A enhancer was detected in two cousins who had pancreatic agenesis. One child with PNDM was heterozygous for the novel p.H410Y ABCC8 variant, inherited from his asymptomatic mother, and another PNDM was heterozygous for the known missense mutation p.R201C in KCNJ11. This mutation was not detected in his consanguineous parents and was therefore likely to have arisen de novo. In the PNDM patients without a genetic cause identified, analysis of the coding regions and conserved splice sites of all the known neonatal diabetes genes did not detect a mutation. One patient with TNDM had loss of methylation on the maternal allele at 6q24 and the other was homozygous for the INS c.-331C>G mutation. This INS mutation was associated with PNDM in other family members and with later diabetes onset in two subjects from a different family diagnosed at 13 and 18 years of age. A third patient with late presentation at 14 months had a homozygous EIF2AK3 mutation.
Phenotype and Clinical Management
The phenotype of the NDM patients and three subjects diagnosed after age 6 months are summarized in Table I.
All 25 patients with NDM were born small for gestational age and presented with diabetes at a mean age of 5 weeks (range from 1 hr to 25 weeks). All except three were born to consanguineous parents and 44% (11/25) had extra-pancreatic features. Of the 23 PNDM patients, 10 (43.4%) had isolated PNDM and three had pancreatic aplasia on imaging. Twins were delivered at 31 weeks while the rest were born at term. The eldest surviving patient was 30 years and 7/20 (31%) patients died of non-diabetes illnesses. All surviving PNDM patients except one are still on insulin.
Mutations in EIF2AK3 causing Wolcott-Rallison syndrome (WRS) were identified in 39.1% (9/23) of our PNDM patients. We recently described the details of these patients within a large WRS series [Habeb, 2015]. In summary, all patients had diabetes, liver disease, and short stature. Neutropenia was observed in eight children and seven needed intermittent courses of erythropoietin for normochromic normocytic anemia. Five patients had skeletal dysplasia (SD), two needed multiple surgeries and one was wheelchair-ridden. Five patients died of acute fulminant hepatitis while patient 2.1 was rescued by liver transplantation (LT) at 2.4 years. He was the only child in this cohort to present beyond the age of 6 months and 6 years post LT he had better diabetes control with no liver disease. However his SD deteriorated resulting in severe spine and lower limbs deformities. Phenotypic variation in the onset and severity of WRS features was demonstrated among six affected members of an extended family (1.1–6; Fig. 2). In this family, diabetes control was variable despite intensive insulin therapy and was complicated by recurrent unpredictable hypoglycemic convulsions which often did not respond to glucagon. Recently, the parents of patient 1.3 opted for pre-implantation Genetic Diagnosis (PGD) for WRS and delivered a healthy girl.
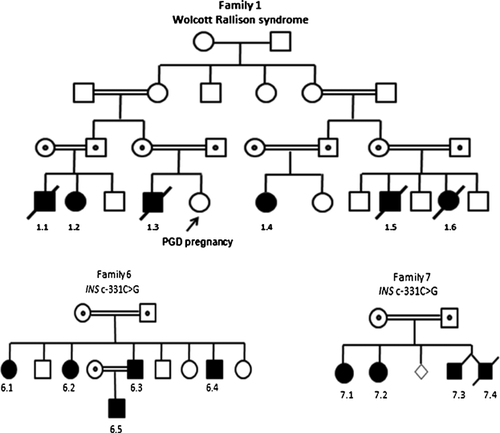
The nine patients with the INS c.-331C>G mutation were from two unrelated families. They had isolated diabetes with variability in the age of onset, duration of disease and insulin requirement even between siblings of the same gender. Four of the five affected members of family 6 (Fig. 2) were initially reported in 2010 [Garin, 2010]: two presented at 6 weeks while the other two developed diabetes at 13 and 18 years. Currently, all are young adults and on insulin. Another family member (a son of 6.3) was subsequently diagnosed with a plasma glucose level of 28 mmol in the first hour of life and currently needs an insulin dose of 1.5 units/kg in order to maintain areasonable glycemic control. All four affected members of family 7 (Fig. 2) presented with hyperglycemia on day 2 of life. However, patient 7.1 remitted at 6 weeks and remains off insulin at the current age of 6 years. In contrast, his 5 years old sister (7.2) still needs 1 mg/kg/day insulin MDI to maintain her HbA1c between 8–9%. One of twins (7.4) was started on insulin but died at 2 weeks because of sepsis while the other (7.3) remains on insulin at age 22 months.
Patient 8.1 presented with diabetic ketoacidosis at 6 weeks of age. She was born to consanguineous parents and found to be heterozygous for the KCNJ11 p.R201C mutation without neurological features. She was switched from insulin to oral sulphonylurea (SU) at 9 months and was off insulin after 5 weeks with a dose of Glibenclamide of 1.1 mg/kg/day 12 hourly. She had persistent diarrhea on the initial few days of treatment with SU requiring hospital admission. On an increased SU dose she developed postprandial hypoglycemia which improved gradually. Eight weeks after withdrawal of insulin her HbA1c was 6.4% compared to 10–12% while on insulin. Currently she is on Glibenclamide 0.4 mg/dg/day in two divided doses, has normal growth and age appropriate developmental milestones. Patient 9.1 had isolated PNDM and was heterozygous for a novel ABCC8 variant, p.H410Y. He has normal growth and intelligence and has not been tried on SU. At the age of 17 years, his insulin requirement is over 1 unit/kg/day on insulin pump therapy. Interestingly his 45-year-old mother, who is a carrier for this variant, has glucose intolerance with HbA1c of 5.9% on diet control.
The child with 6q24 TNDM (10.1) presented with hyperglycemia at 6 weeks of age and remitted 3 weeks later. He relapsed at 11 weeks requiring intermittent insulin for 18 months. He is currently 8 years old and has been off insulin. He has global developmental delay, brain atrophy and dysmorphic features (Table I). In addition he has nystagmus, agenesis of corpus callosum, and absent septum pellucidum.
Patients 11.1 and 11.2 are first degree cousins. Both presented with neonatal diabetes and features of pancreatic agenesis on MRI. Deletion of the PTF1A enhancer was detected in the younger cousin. All four children without identifiable genetic cause in the cohort had PNDM. Patient 12.1 had early features of exocrine pancreatic deficiency with absent pancreas confirmed on CT scan. He was managed by dietary support, pancreatic enzyme supplement, and intensive diabetes treatment via insulin pump. Subject 15.1 had Down syndrome confirmed by chromosomal analysis and developed non-autoimmune diabetes at 4 months. The remaining two patients (13.1 and 14.1) had isolated PNDM with good control on insulin.
DISCUSSION
We report the first data on NDM from the Emirate of AbuDhabi in the UAE. In this cohort, 84% of patients had identifiable genetic etiology with recessive EIF2AK3 mutations being the most frequent cause, followed by homozygous INS mutations. 92% of the 25 patients had PNDM and 44% showed extra-pancreatic features. The incidence of NDM in Emirates of Abu Dhabi during 1985–2013 was 1 in 29,241, which is three times the highest reported rate in Europe [Grulich-Henn, 2010]. Our PNDM incidence of 1:31,900 is comparable to the incidence of 1:48,000 reported in South East Turkey [Demirbilek, 2015] and 1 in 21,000 from Northwest Saudi Arabia [Habeb, 2012a]. However it is more than six times the incidence of PNDM in Jordan [Abujbara, 2014], the USA [Kanakatti, 2013], and three European countries [Slingerland, 2009]. We suspect that the main reason for the high incidence of this monogenic condition in our area is the combination of high consanguinity and birth rate. Of note, all except three patients were born to related parents and three of our 15 families had at least four affected members.
Earlier reports on NDM from the Gulf region focused mainly on PNDM [Bappal, 1999, Habeb, 2012a,2012b]. In our series two children had TNDM making a total number of six TNDM patients from the Gulf region reported with TNDM to date [Dib, 1995; Attia, 1998]. It is possible that in this region, TNDM is less frequent, under diagnosed or under-reported. One of our two patients with TNDM had methylation defect of 6q24 and showed most congenital anomalies described with this neonatal diabetes subtype [Diatloff-Zito, 2007; Docherty, 2013]. However, to our knowledge, his midline brain defect has not been previously reported in 6q24 TNDM. The other TNDM patient was the only of our nine patients with homozygous INS c-331C>G mutation to have TNDM. This mutation is located in the promoter region of INS and was previously found to cause TNDM in one subject in the series published by Garin, [2010]. All patients with this mutation had low birth weight in keeping with reduced insulin secretion in utero; however there was a variability in the age of onset, severity and duration of diabetes even between siblings of the same gender. Strikingly, in family 6, a boy developed hyperglycemia in the first hour of life while his father presented at 18 years and in family 7, a boy had TNDM while his 5 year old sister was still on insulin. The exact cause for this variation is unclear. Various experimental/epidemiological studies demonstrate profound influence of maternal stressors and intrauterine environment on gene expression in the placenta [Ciprian, 2010]. We speculate that factors such as epigenetic variation, intrauterine stress, or the presence of unidentified modifier genes may contribute to the phenomenon of the variability of gene expression.
WRS was the commonest cause of PNDM in our cohort as reported in other consanguineous populations [Habeb, 2012a]. This syndrome is caused by recessive EIF2AK3 mutations and characterized by early onset diabetes, intermittent hepatitis, and skeletal dysplasia. Consistent with other reports [Ozbek, 2010; Al-Shawi, 2013; Habeb, 2013], there was variability in the phenotype in the 10 patients. Most patients with WRS die during childhood with AFH and the mortality rate in our cohort was 60%, which is similar to rates reported from KSA and Turkey [Ozbek, 2010; Habeb, 2013]. The encouraging results of our first patient to undergo LT [Habeb, 2015] and the recent report of successful liver, pancreas, and kidney transplant in another child with WRS [Tzakis, 2015] raises the hope for a better life expectancy for these children. Similar to other inherited conditions with poor prognosis, genetic counseling is a cornerstone in the management of WRS. Advances in reproductive medicine enable parents at high risk of monogenic conditions to have unaffected children by selecting healthy embryos using PGD [Brezina, 2015]. This technique is less invasive than antenatal genetic diagnosis via chorionic villous sampling and avoids the need for termination of pregnancy, which is not easily acceptable in certain cultures. It provides an opportunity for carriers of recessive conditions to have a healthy child. We report the first parents of a WRS patient to have a healthy girl following PGD. The positive experience of this family would open the door for other WRS families to have healthy children.
KATP mutations (KCNJ11 and ABCC8) are the commonest cause of PNDM in outbred populations; however they were not found to be a common cause of PNDM in Arabs [Habeb, 2012b] or consanguineous probands with PNDM tested in Exeter [Rubio-Cabezas, 2013]. In our PNDM series 2/23 (8%) had KATP mutations. Given the frequency of these mutations in Europe this number is expected for our cohort of 701,000 live births. In our patient, the heterozygous KCNJ11 mutation was not detected in her related parents indicating that the mutation is either de novo or one of the parents may have germline mosaicism. Prior to this girl only one child with a KCNJ11 mutation from the Gulf region was reported to be successfully switched from SU to insulin [Al-Mahdi, 2010]. However that child was born to an unrelated couple. The fact that our patient was born to first cousins highlights the importance of testing for KCNJ11 mutations even in NDM patients born to consanguineous parents and in populations where KATP mutations are not a common cause of NDM. It is not clear whether the ABCC8 p.H410Y variant identified in the patient 9.1 is pathogenic; a functional study is needed to clarify this point. A trial of SU following the functional study results will be considered.
We were able to define the genetic cause of diabetes in more than 80% of our NDM patients which is similar to rates reported in other studies of highly consanguineous populations [Habeb, 2012a; Demirbilek, 2015]. Although some patients were diagnosed with diabetes long time ago, the genetic diagnosis of NDM was confirmed recently perhaps reflecting easier access to genetic testing and better awareness by clinicians of the importance of genetic diagnosis in the management of NDM. The clinical utilities of genetic testing in our cohort were illustrated in a number of families; first it enabled us to make the diagnosis of neonatal diabetes due to a heterozygous KCNJ11 mutation and the child subsequently achieved better glycemic control following the transfer from insulin to oral SU. Second, the detection of a recessive EIF2AK3 mutation in patient 1.4 with WRS enabled his heterozygous parents to have a healthy child through the PGD. Finally, the identification of homozygous INS mutation in the proband of family 6 revised the diagnosis of diabetes in his siblings who had been diagnosed with T1DM and led to early detection of the cause of hyperglycemia in one of the offspring in the first hour of life.
Our study has two limitations: first it does not reflect the whole country but given the high birth rate and consanguinity in other parts of the UAE, a similar picture is expected. Second, patients were identified retrospectively from follow-up records of diabetes clinics; it is therefore possible that some patients were missed either due to moving to different areas or dying before the diagnosis of NDM was established. Clearly, establishing a national diabetes registry will provide more accurate figures on NDM in the whole country. In summary, in AbuDhabi, TNDM is rare. However, the frequency of PNDM is among the highest in the world and its etiology is different from Europe and the USA, but similar to Northwest Saudi Arabia and Turkey. The results of genetic testing had significant implication on clinical management of our NDM patients.
ACKNOWLEDGMENTS
We acknowledge Dr. Ahmed Massoud for including one of his patients in the cohort, Koreena Sheath for her help in identifying patients referred from AbuDhabi in Exeter database, and Jane Houghton for her contribution in the genetic analyses.




