Clinical and genetic characteristics of craniosynostosis in Hungary
Abstract
Craniosynostosis, the premature closure of cranial sutures, is a common craniofacial disorder with heterogeneous etiology and appearance. The purpose of this study was to investigate the clinical and molecular characteristics of craniosynostoses in Hungary, including the classification of patients and the genetic analysis of the syndromic forms. Between 2006 and 2012, 200 patients with craniosynostosis were studied. Classification was based on the suture(s) involved and the associated clinical features. In syndromic cases, genetic analyses, including mutational screening of the hotspot regions of the FGFR1, FGFR2, FGFR3, and TWIST1 genes, karyotyping and FISH study of TWIST1, were performed. The majority (88%) of all patients with craniosynostosis were nonsyndromic. The sagittal suture was most commonly involved, followed by the coronal, metopic, and lambdoid sutures. Male, twin gestation, and very low birth weight were risk factors for craniosynostosis. Syndromic craniosynostosis was detected in 24 patients. In 17 of these patients, Apert, Crouzon, Pfeiffer, Muenke, or Saethre–Chotzen syndromes were identified. In one patient, multiple-suture craniosynostosis was associated with achondroplasia. Clinical signs were not typical for any particular syndrome in six patients. Genetic abnormalities were detected in 18 syndromic patients and in 8 relatives. In addition to 10 different, known mutations in FGFR1,FGFR2 or FGFR3, one novel missense mutation, c.528C>G(p.Ser176Arg), was detected in the TWIST1 gene of a patient with Saethre–Chotzen syndrome. Our results indicate that detailed clinical assessment is of paramount importance in the classification of patients and allows indication of targeted molecular testing with the highest possible diagnostic yield. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Craniosynostosis, characterized by premature sutural fusion, is a congenital disorder with an estimated prevalence of 1 in 2,100–2,500 births [Lajeunie et al., 1995; Boulet et al., 2008]. Cranial morphology can vary depending on the type and number of sutures involved. In many cases, early surgical intervention is essential to allow appropriate growth of the skull and reduce the possibility of neurological complications. The etiology and presentation of craniosynostosis are rather heterogeneous. In the majority of patients, it is an isolated (nonsyndromic) condition, whereas in the syndromic forms, the cranial abnormality is associated with various additional clinical signs (e.g., facial dysmorphism or limb anomalies). Both environmental and genetic factors may contribute to the premature fusion of sutures. The main risk factors include sex, ethnicity, plurality, birth presentation, birth weight, gestational age, mode of delivery, and maternal or paternal age [Alderman et al., 1988; Singer et al., 1999; Gill et al., ]. Teratogenic exposure, medication use, smoking and alcohol use may also predispose to craniosynostosis [Higginbottom et al., 1980; Källen and Robert-Gnansia, 2005; Carmichael et al., 2008]. Different sutures are related to different risk factors for synostosis; for example, male is associated with sagittal synostosis, but is less of a risk factor for coronal synostosis, and maternal smoking has the strongest association with the premature fusion of the sagittal suture [Källén, 1999; Boulet et al., 2008]. As the prevalence of risk factors can be different in populations, and may change over time, the percentage of the specific sutures involved in craniosynostosis may vary [Lee et al., 2012].
In genetically determined forms, gene mutations or chromosome abnormalities can be identified. Heterozygous mutations in the genes of fibroblast growth factor receptor 1, 2, and 3 (FGFR1, FGFR2 and FGFR3) and the twist family basic helix-loop-helix transcription factor 1 (TWIST1) are the most frequent causes of the syndromic forms. FGFR2 mutations, principally identified in the hotspot regions (exon IIIa and IIIc) of the gene, are involved in several craniosynostosis syndromes including Antley–Bixler, Apert, Jackson–Weiss, Crouzon, and Pfeiffer syndromes [Kan et al., 2002]. A specific mutation, p.Pro252Arg, in the FGFR1 gene can be identified in Pfeiffer syndrome type 1 [Muenke et al., 1994]. The FGFR3 p.Pro250Arg mutation is associated with Muenke syndrome, whereas p.Ala391Glu in the same molecule results in Crouzon syndrome with acanthosis nigricans [Meyers et al., 1995; Muenke et al., 1997]. TWIST1 mutations can be predominantly detected in Saethre–Chotzen syndrome [Howard et al., 1997]. FGFR mutations are gain-of-function mutations, whereas TWIST1 mutations result in haploinsufficiency. These syndromes, similarly to the majority of craniosynostosis syndromes, show an autosomal dominant pattern of inheritance. A wide range of chromosomal abnormalities have been associated with craniosynostosis, most of them are submicroscopic in size [Jehee et al., 2008; Wilkie et al., 2010]. Deletion or translocation of the 7p21 region including the TWIST1 gene is the best characterized chromosomal cause of craniosynostosis [Johnson et al., 1998].
The aim of our work was to investigate the clinical and genetic aspects of patients with craniosynostoses at the main craniofacial surgery center in Hungary in the interval 2006–2012, including the classification of patients (based on the suture involved and the associated clinical features), the genetic analysis of the syndromic forms, and the assessment of selected potential risk factors for craniosynostosis.
MATERIALS AND METHODS
Patients
Two hundred patients with craniosynostosis were referred to the Department of Neurosurgery and Department of Pediatrics, University of Debrecen, the main craniofacial surgery center in Hungary between the years 2006 and 2012. Synostosis of the sutures was confirmed by either X-ray or computed tomography, and the syndromic nature of the disease was established by a team of pediatricians and clinical geneticists. Of the 200 enrolled patients, 198 were under 10 years of age with a median age of 6 months, whereas only two patients were adults (18 and 28 years old). Reconstructive operative procedures were performed in 195 patients. In 24 patients, clinical signs other than the malformed skull suggested the syndromic forms of the disease. In five syndromic patients, the disease was familial. Patients diagnosed with craniosynostosis syndromes during the 7 years of the study had the following types: Apert (n = 5), Crouzon (n = 2), Pfeiffer (n = 5), Muenke (n = 4), and Saethre–Chotzen (n = 1) syndromes. Multiple-suture craniosynostosis associated with achondroplasia was found in one patient (n = 1). Phenotypic features were not typical for any particular syndrome in six patients.
Clinical signs in patients with Apert syndrome included brachycephaly due to bicoronal synostosis, high and broad forehead, midface hypoplasia, depressed nasal bridge, convex nasal ridge, hypertelorism, and syndactyly of fingers and toes. In the majority of patients, syndactyly of all toes on both feet and syndactyly of the 2/3/4/5 fingers on both hands were observed (Patients 1–4). In Patient 5, complete syndactyly of all fingers and all toes was seen. Cleft palate was observed in Patient 1.
Both Crouzon syndrome patients had brachycephaly, proptosis, flat nasal bridges, mandibular prognathism, and low-set ears (Patients 6 and 7). In Patient 7, acanthosis nigricans was also present.
Three Pfeiffer syndrome patients (Patients 8–10) had severe cranial malformation (cloverleaf or “Kleeblattschädel” skull), hydrocephalus, extreme proptosis, low-set ears, and short, small noses. Broad great toes were observed in all of these patients, whereas the thumbs were broad only in Patients 9 and 10. Limited extension of the elbows was noticed in Patients 8 and 10. In Patient 9, medially deviated great toes, the characteristic feature of Pfeiffer syndrome, were observed and were associated with partial 2/3 syndactyly on the feet. Based on the phenotypic signs, the latter three patients were considered to have Pfeiffer syndrome type 2. In two other patients, Pfeiffer syndrome type 1 was diagnosed based on craniofacial anomalies milder than those in patients with type 2. Patient 11 had brachycephaly, a high and broad forehead, exophthalmos, broad thumbs, 3/4 syndactyly of the fingers, broad great toes, and 2/3/4 syndactyly of the toes. Clinical and genetic investigations of Patient 12 and her family members have been published recently [Bessenyei et al., 2014].
All of the Muenke syndrome patients had brachycephaly resulting from the synostosis of the coronal sutures, high and flat foreheads, hypertelorism, and almond-shaped eyes. High-arched palate was seen in two of them (Patients 13 and 14). Limb alterations were variable, including broad thumbs and great toes (Patient 14 and 16), clinodactyly of the fifth fingers (Patient 13) and capitate–hamate fusion (Patient 15). The mothers of Patients 13, 15, and 16 showed similar phenotypes to those of their children, but their clinical signs were milder. The parents of Patient 14 and the sibling of Patient 15 were not available for the clinical and genetic examination.
Typical clinical signs of Saethre–Chotzen syndrome were observed in Patient 17, who had brachycephaly, an asymmetric flat face, a long and deviated nose, thin lips, ptosis of the right eyelid, shallow orbits, and broad and bifid great toes, suggesting the Robinow–Sorauf variant of the syndrome. The mother of the patient also had brachycephaly, a long nose, thin lips, and bifid great toes; additionally, her thumbs were broad.
Multiple-suture craniosynostosis associated with rhizomelic shortening of the limbs and trident hands, the typical clinical signs of achondroplasia was found in Patient 18.
In six patients, additional clinical features associated with craniosynostosis included various facial dysmorphic signs and limb alterations suggesting the syndromic form of craniosynostosis; however, the phenotypes of these patients were not typical for any particular syndrome. Patient 19 had an asymmetric face due to left anterior plagiocephaly, short stature, broad thumbs, and a single transverse palmar crease. Patient 20 had brachycephaly, a flat occiput, low hair-line, upslanted palpebral fissures, a high arched palate, supernumerary teeth, and partial 2/3 syndactyly of toes. Turricephaly, high and flat forehead, blepharophimosis, long philtrum, mild mandibular prognathism, broad great toes, and partial 2/3 syndactyly of the toes on the left foot characterized Patient 21. Patient 22 had hypertelorism, exophthalmos, and low-set ears. Patient 23 had a prominent occiput, a high and broad forehead, proptosis, strabismus, a short philtrum, macroglossia, low-set ears, and broad great toes. Patient 24 had trigonocephaly and partial 2/3 syndactyly of the fingers.
Molecular and Cytogenetic Analyses
Genetic analyses were performed only for patients with clinically identified or suspected syndromic craniosynostosis and relatives showing the clinical signs of a specific syndrome. After obtaining written informed consent, blood samples were taken from 24 syndromic patients and 8 relatives. The diagnostic strategy was based on the study of Johnson and Wilkie [2011]. Genetic testing included the molecular analysis of mutational hotspots in the FGFR1, FGFR2, FGFR3, and TWIST1 genes, G-banded karyotyping and fluorescence in situ hybridization (FISH) analysis of TWIST1. If a specific syndrome was identified, targeted analysis was performed, whereas in suspected syndromic cases, all of the previously mentioned tests were applied.
Genomic DNA was extracted from the patients’ peripheral blood using the QIAamp DNA Blood Mini kit (Qiagen, Germantown, MD), and amplification of exon 7 (p.Pro252Arg site) of FGFR1, exons 8 (IIIa) and 10 (IIIc) of FGFR2, exons 7 (p.Pro250Arg site) and 10 (p.Ala391Glu site) of FGFR3, and the coding region of exon 1 in TWIST1 was performed by polymerase chain reaction (PCR) with previously published primer pairs [Paznekas et al., 1998; Kan et al., 2002; Baroni et al., 2005; Seto et al., 2007]. For DNA sequencing, the PCR products were purified using the MinElute PCR Purification Kit (Qiagen). Purified PCR products were sequenced on the ABI 3100 sequencer (Applied Biosystems, Foster City, CA) with the Big Dye Terminator v3.1 cycle sequencing kit (Applied Biosystems). Electropherograms of the sequenced products were compared to reference sequences (FGFR1, NG_007729; FGFR2, NG_012449; FGFR3, NG_012632; and TWIST1, NG_008114).
Karyotyping based on GTG banding was performed on metaphase cells after culturing of peripheral blood lymphocytes using standard methods. To investigate TWIST1 gene deletion or translocation, FISH analysis was performed on cell suspension derived from chromosome preparation using the Saethre–Chotzen/Williams–Beuren Probe (Cytocell, Cambridge, UK) according to the manufacturer's instruction.
Assessing the Pathogenicity of the Novel Variant
To assess the pathogenicity of the novel variant, c.528C>G(p.Ser176Arg) in exon 1 of TWIST1, SIFT, and Polyphen-2 predictions were performed [Ng and Henikoff, 2001; Adzhubei et al., 2010]. Alignment analysis of TWIST1 proteins was carried out using ClustalOmega [Sievers et al., 2011]. The presence of the novel variant in 50 healthy individuals was tested by RFLP analysis using BspMI restriction enzyme. A partial sequence of exon 1 of the TWIST1 gene was amplified as a 512-bp product. In the case of the wild-type allele, the BspMI does not digest the PCR products. In the presence of the mutant allele, digestion with BspMI yields 295-bp and a 217-bp restriction fragments.
Statistical Analysis
To assess the potential risk factors for craniosynostosis, selected perinatal data (sex, plurality, gestational age, and birth weight) of 142 nonsyndromic patients were compared with the general Hungarian live birth data (average data from the years 2005 to 2010) provided by the Hungarian Central Statistical Office [Vukovich, 2011]. Statistical analyses included Chi-square or Fisher's exact tests performed by IBM SPSS Statistics 20.0 (IBM Corporation, Armonk, NY). A P-value <0.05 was considered statistically significant.
RESULTS
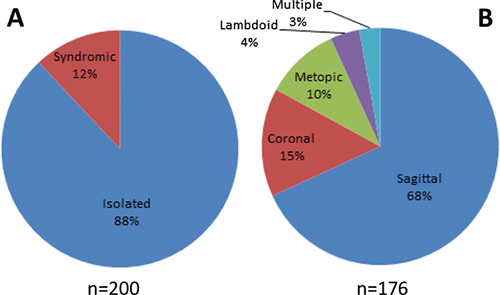
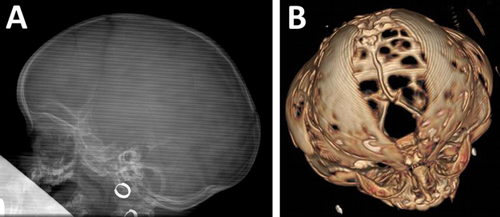
Our study comprised 200 patients with craniosynostosis. On the basis of detailed clinical assessment, the condition proved to be isolated (nonsyndromic) in 176 (88%) patients and syndromic in 24 (12%) patients. The male-to-female ratio was 2.2:1, showing a male predominance. In the isolated group, the most frequently fused suture was the sagittal one (120/176; 68%) followed by the coronal (26/176; 15%), metopic (18/176; 10%), and lambdoid sutures (7/176; 4%). In five patients (3%), more than one suture was involved (Fig. 1). As FGFR3 gene analysis was not performed in nonsyndromic patients with coronal synostosis, the possibility that some of these patients are affected by Muenke syndrome cannot be ruled out; therefore, the real involvement of the coronal suture may be slightly lower in the isolated group. X-ray and 3D-CT images of the scaphocephal and cloverleaf skull configuration are shown in Figure 2.
Perinatal data of 142 nonsyndromic patients were compared to the general Hungarian live birth data to assess possible associations with craniosynostosis (Table I). Male (P < 0.001), twin gestation (P < 0.001), and very low (<1,500 g) birth weight (P < 0.001) were risk factors for nonsyndromic craniosynostosis. Being male (P < 0.001) and twin gestation (P = 0.001) were associated with sagittal synostosis, whereas very low birth weight was a predictor for coronal (P < 0.001) synostosis. Metopic synostosis was not associated with any factor analyzed in the study. Because of the small number of patients, statistical analysis was not performed on lambdoid synostosis. Seven patients with nonsyndromic craniosynostosis had one of the following additional major defects: ventricular septal defect, atrial septal defect, renal pelvis dilatation, pyloric stenosis, and choanal atresia.
| All nonsyndromic craniosynostosis (n = 142) | Sagittal (n = 100) | Metopic (n = 18) | Coronal (n = 17) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Number of cases | Population controls (%) | Odds ratio | 95% confidence interval | P | Odds ratio | 95% confidence interval | P | Odds ratio | 95% confidence interval | P | Odds ratio | 95% confidence interval | P |
| Sex | ||||||||||||||
| Male | 99 (69.7%) | 51.3 | 2.18 | 1.53–3.12 | <0.001 | 3.36 | 2.09–5.39 | <0.001 | 2.47 | 0.88–6.92 | 0.078 | 0.13 | 0.03–0.55 | 0.005 |
| Female | 43 (30.3%) | 48.7 | ||||||||||||
| Plurality | ||||||||||||||
| Twin | 14 (9.9%) | 3.5 | 3.01 | 1.73–5.23 | <0.001 | 3.06 | 1.59–5.88 | 0.001 | 3.44 | 0.79–14.98 | 0.266 | 0.79 | 0.05–13.09 | 0.899 |
| Singleton | 128 (90.1%) | 96.5 | ||||||||||||
| Gestational age | ||||||||||||||
| Preterm (<37 weeks) | 18 (12.7%) | 8.7 | 1.52 | 0.93–2.50 | 0.125 | 1.16 | 0.61–2.24 | 0.078 | 2.10 | 0.61–7.24 | 0.436 | 2.25 | 0.65–7.82 | 0.381 |
| Term (37–41 weeks) | 124 (87.3%) | 91.3 | ||||||||||||
| Birth weight | ||||||||||||||
| Very low (<1,500 g) | 8 (5.6%) | 1.4 | 4.12 | 2.01–8.45 | <0.001 | 2.79 | 1.02–7.61 | 0.094 | 4.94 | 0.64–37.99 | 0.537 | 13.67 | 3.89–48.02 | <0.001 |
| Low (1,500–2,499 g) | 9 (6.3%) | 6.9 | 0.93 | 0.47–1.84 | 0.978 | 0.70 | 0.29–1.73 | 0.566 | 1.99 | 0.45–8.89 | 0.676 | 0.44 | 0.06–7.44 | 0.600 |
| Normal (2,500–3,999 g) | 115 (81.0%) | 83.0 | ||||||||||||
| Macrosomia (≥4,000 g) | 10 (7.1%) | 8.7 | 0.83 | 0.43–1.58 | 0.677 | 0.67 | 0.29–1.54 | 0.445 | 2.38 | 0.67–8.45 | 0.343 | 0.73 | 0.09–5.61 | 0.764 |
In the syndromic group, the Apert and Pfeiffer syndromes were most commonly observed. Genetic abnormalities were detected in 75% (18/24) of the syndromic patients and in eight relatives showing the clinical signs of a specific syndrome (Table II).
| Patient | Syndrome | Gene | Nucleotide change | Effect | Number of relatives with mutation |
|---|---|---|---|---|---|
| 1 | Apert | FGFR2 | c.755C>G | p.Ser252Trp | |
| 2 | Apert | FGFR2 | c.758C>G | p.Pro253Arg | |
| 3 | Apert | FGFR2 | c.758C>G | p.Pro253Arg | |
| 4 | Apert | FGFR2 | c.758C>G | p.Pro253Arg | |
| 5 | Apert | FGFR2 | c.758C>G | p.Pro253Arg | |
| 6 | Crouzon | FGFR2 | c.833G>T | p.Cys278Phe | |
| 7 | Crouzon | FGFR3 | c.1172C>A | p.Ala391Glu | |
| 8 | Pfeiffer | FGFR2 | c.1024T>C | p.Cys342Arg | |
| 9 | Pfeiffer | FGFR2 | c.1024T>C | p.Cys342Arg | |
| 10 | Pfeiffer | FGFR2 | c.1025G>C | p.Cys342Ser | |
| 11 | Pfeiffer | FGFR2 | c.940-1G>A | Splicing defect | |
| 12 | Pfeiffer | FGFR1 | c.755C>G | p.Pro252Arg | 4 |
| 13 | Muenke | FGFR3 | c.749C>G | p.Pro250Arg | 1 |
| 14 | Muenke | FGFR3 | c.749C>G | p.Pro250Arg | |
| 15 | Muenke | FGFR3 | c.749C>G | p.Pro250Arg | 1 |
| 16 | Muenke | FGFR3 | c.749C>G | p.Pro250Arg | 1 |
| 17 | Saethre–Chotzen | TWIST1 | c.528C>G | p.Ser176Arg | 1 |
| 18 | Achondroplasia | FGFR3 | c.1138G>A | p.Gly380Arg |
In addition to 10 different known mutations detected in FGFR1–3, one previously undescribed missense mutation, c.528C>G(p.Ser176Arg), was found in the TWIST1 gene of Patient 17. The pathogenicity of this mutation is supported by the following facts: (1) the affected residue is phylogenetically highly conserved in human, mouse, zebrafish, and xenopus; (2) according to SIFT and PolyPhen-2 predictions, this missense alteration was predicted to be damaging and probably damaging, respectively; (3) RFLP analysis of exon 1 of TWIST1 showed that this mutation was not present in 50 healthy individuals representing 100 alleles. The mother, who had very similar phenotype also had the novel c.528C>G(p.Ser176Arg) mutation.
In Patient 18, molecular analysis detected the achondroplasia-specific c.1138G>A(p.Gly380Arg) mutation. Clinical and genetic investigation of this patient has been previously reported in detail [Bessenyei et al., 2013].
Syndromic craniosynostosis was identified in a further six patients; however, their clinical signs were not specific for any particular craniosynostosis syndrome. Mutational analysis of the hotspot regions of FGFR1–3 and TWIST1 did not reveal any genetic alteration. Conventional G-banded karyotyping and TWIST1 FISH analysis also showed normal results in these patients.
DISCUSSION
Our study is the first nation-wide investigation providing clinical and genetic information on craniosynostoses in Hungary. Similar to previously published studies, nonsyndromic patients represented the majority of all cases with craniosynostosis, and the most frequently involved suture was the sagittal one, followed by the coronal, metopic, and lambdoid sutures [Wilkie et al., 2010; Kolar, 2011]. The frequency of sagittal synostosis was found to be 68%, which is higher than in other populations. Assessing the relationship between potential risk factors and craniosynostosis, it was found—in accordance with previous studies—that sagittal synostosis was associated with male and twin gestation [Boulet et al., 2008; Lee et al., 2012]. Because the prevalence of these factors is not significantly higher in the general Hungarian population than in other populations, the associations above cannot be the cause of the higher frequency of sagittal synostosis. Several studies confirmed the role of maternal smoking as a risk factor for craniosynostosis, especially the sagittal type [Honein and Rasmussen., 2000; Carmichael et al., 2008; Hackshaw et al., 2011]. According to data from the World Health Organization (2011), the estimated prevalence of daily smoking in Hungarian females is 26%, which is much higher than in many other countries (United Kingdom, 14%; United States, 13%; Australia, 15%). Although smoking behavior of mothers was not studied in our patient cohort, we can speculate that smoking might be one of the potential causes of the higher involvement of the sagittal suture in Hungarian patients with craniosynostosis.
Genetic abnormalities were detected in syndromic patients with clinical signs characteristic for a specific syndrome. According to the literature, the specific mutations p.Ser252Trp and p.Pro253Arg of FGFR2 can be detected in about 99% of patients with Apert syndrome, whereas Alu-element insertions in FGFR2 are a rare cause of this condition [Oldridge et al., 1999]. In our study, all patients with Apert syndrome carried one of the two specific mutations. Interestingly, the p.Ser252Trp mutation could be detected in only one patient, whereas other studies found this mutation with a higher frequency [Park et al., 1995; Oldridge et al., 1999]. Cleft palate has been reported to be more common with the p.Ser252Trp mutation, whereas syndactyly was found to be more severe in patients with the p.Pro253Arg mutation [Slaney et al., 1996]. Our result supports the former observation: the only patient, who had cleft palate, carried the p.Ser252Trp mutation.
Mutations eliminating the cysteine 342 residue in FGFR2 have a different phenotypic impact. Its conversion to phenylalanine or tyrosine mainly results in Crouzon syndrome, whereas arginine substitution preferentially causes Pfeiffer syndrome with severe cranial manifestation and poor prognosis [Lajeunie et al., 2006]. In our cohort, two patients with Pfeiffer syndrome harbored the p.Cys342Arg mutation; both of them had cloverleaf skulls requiring two and three reconstructive surgical interventions, hydrocephalus, severe exophthalmos, and respiratory and auditory problems. In addition, exchange of the same amino acid residue for serine caused very similar, severe phenotype in our third patient with Pfeiffer syndrome type 2.
A specific mutation of FGFR1, p.Pro252Arg, is a very rare cause of craniosynostosis predominantly associated with Pfeiffer syndrome [Muenke et al., 1994]. Phenotypic features in the family with that mutation showed high variability ranging from apparently normal skull and limbs to characteristic brachycephaly and digital anomalies. Typical features of the syndrome appeared only in the third generation, suggesting that this condition is underdiagnosed in many cases.
We identified a novel missense mutation, p.Ser176Arg, in TWIST1 in a patient with Saethre–Chotzen syndrome. TWIST1 is a basic helix-loop-helix transcription factor involved in a variety of signal transduction pathways in tissues of mesodermal origin. It has a crucial role in the migration and differentiation of cranial neural crest cells during cranial development [Soo et al., 2002]. The novel alteration is located between the highly conserved bHLH and Twist box domains. Although this linker region is evolutionarily conserved across a wide range of vertebrate species, its functional importance is still unclear.
Although the clinical signs are heterogeneous and overlap between the different forms because of, among others, the variable expressivity, the leading symptoms of the specific craniosynostosis syndromes allow the indication of targeted molecular tests. Our results suggest that clinical genetic assessment and genetic testing of patients are not of great importance in the delineation of subtypes, classification of patients, and risk assessment in the affected families, they also allow early surgical intervention and contribute to the knowledge of the prognosis. The complex care of these patients needs a multidisciplinary approach, a team consisting of pediatricians, geneticists, neurosurgeons, imaging specialists, and laboratory experts in close collaboration with each other.