Co-occurrence of hypertrophic cardiomyopathy and myeloproliferative disorder in a neonate with Noonan syndrome carrying Thr73Ile mutation in PTPN11
Abstract
Most cases of Noonan syndrome (NS) result from mutations in one of the RAS-MAPK signaling genes, including PTPN11, SOS1, KRAS, NRAS, RAF1, BRAF, SHOC2, MEK1 (MAP2K1), and CBL. Cardiovascular diseases of varying severity, such as pulmonary stenosis and hypertrophic cardiomyopathy (HCM), are common in NS patients. RAF1 mutations are most frequent in NS with HCM, while PTPN11 mutations are also well known. Thr73Ile is a gain-of-function mutation of PTPN11, which has been highly associated with juvenile myelomonocytic leukemia and NS/myeloproliferative disease (MPD), but has not previously been reported in HCM. Here, we report a Japanese female infant with NS carrying the PTPN11 T73I mutation with NS/MPD, complete atrio-ventricular septal defect, and rapidly progressive HCM. No other HCM-related mutations were detected in PTPN11, RAF1, KRAS, BRAF, and SHOC2. This patient provides additional information regarding the genotype–phenotype correlation for PTPN11 T73I mutation in NS. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Noonan syndrome (NS: OMIM #163950) was first reported in 1962 in patients with congenital heart disease [Noonan, 1968]. It is characterized by distinctive facial dysmorphism, cardiac anomalies, short stature, webbed neck, cryptorchidism, and, rarely, with hematologic malignancies during childhood [Roberts et al., 2013]. NS exhibits phenotypic overlap with Costello syndrome and cardio-facio-cutaneous syndrome. Most cases are the result of germline mutations in RAS-MAPK signaling genes such as PTPN11, SOS1, KRAS, NRAS, RAF1, BRAF, SHOC2, MEK1 (MAP2K1), and CBL. NS has recently been referred to as “RASopathies” or “RAS/MAPK syndrome” [Rauen et al., 2010].
RAF1 mutations have frequently been reported in genetic analyses of NS patients with hypertrophic cardiomyopathy (HCM), and PTPN11 mutations are also positively associated with pulmonary stenosis, ASD, and HCM [Prendiville et al., 2014]. Thr73Ile is a gain-of-function mutation in PTPN11 that results in excessive Src homology 2-containing phosphotyrosine phosphatase (SHP2) activity. This mutation is highly associated with juvenile myelomonocytic leukemia and NS/myeloproliferative disease (MPD) [Kratz et al., 2005], but has not previously been reported in a patient with HCM. Here, we report the case of an infant with NS carrying the PTPN11 T73I mutation who presented with MPD, complete atrio-ventricular septal defect (CAVSD), and HCM.
CLINICAL REPORT
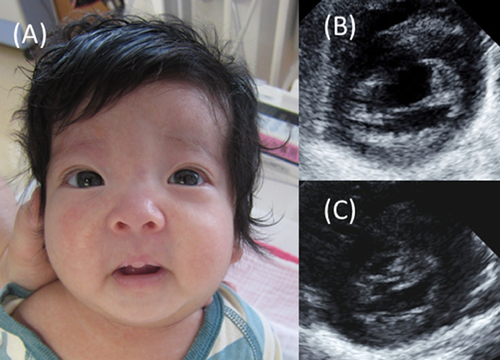
The female infant was born as a second child to healthy, non-consanguineous parents at 38 weeks’ gestation, weighing 2,696 g. Her mother was introduced to our hospital at 25 weeks’ gestation because of suspected congenital heart disease in her infant. Amniotic examination revealed a normal karyotype. After delivery, the infant was admitted to the neonatal intensive care unit with peripheral cyanosis. Her pulse rate was regular at 140 bpm, blood pressure was 62/30 mmHg, and pulse oximetry recorded an oxygen saturation of 94%. Physical examination revealed a peculiar face with hypertelorism, blepharophimosis, droopy eyelids, webbed neck, and low-set and posteriorly rotated ears (Fig. 1A). There were no signs of abnormal skin pigmentation or fibromatosis, sensorineural deafness, or abnormal genitalia. Ultrasound examination confirmed CAVSD with a common atrioventricular valve, leading to a diagnosis of CAVSD Rastelli Type A without left ventricular hypertrophy, with normal left ventricular end-systolic dimension (LVSd) of 3.7 mm (Z-score: −0.02), and left ventricular posterior wall dimension (LVPWd) of 2.5 mm (Z-score: −0.43) (Fig. 1B). Peripheral-blood analysis revealed MPD based on a decrease in platelet count from 176,000 to 52,000 mm3, and an increase in monocyte count from 2,050 to 8,490 mm3 during the first week of life. No blast cells were detected in her bone marrow. NS or LEOPARD syndrome was suspected. Genetic analysis revealed a normal karyotype 46, XX, and no deletions at 22q.11.2 by fluorescence in situ hybridization. Further analysis of DNA from peripheral lymphocytes, bone marrow, hair, and nails was performed using Sanger sequencing analysis or target sequencing for inherited hematologic diseases. We identified a c.218C>T, p.T73I mutation in exon 3 of PTPN11 in the patient's lymphocytes, which was confirmed as a somatic mutation by its detection in hair and nail samples. No other significant mutations in PTPN11, or in BRAF, KRAS, NF1, exons 7, 14, 21 of RAF1, exon 5 of RIT1, exon 1 of SHOC2, or exon 2 of HRAS were detected, and the patient was therefore diagnosed with NS.
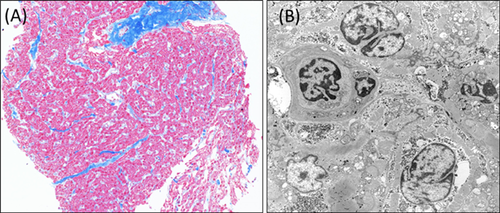
The patient's cardiac function after birth demonstrated pulmonary artery overflow and significant left ventricular hypertrophy, with a LVSd of 7.7 mm (Z-score: 3.52) and LVPWd of 4.5 mm (Z-score: 2.41) at 20 days after birth, with no left ventricular outlet stenosis. Myocardial biopsy at 5 months of age revealed extensive fibrosis, nuclear irregularity, an indistinct array of myocardial cells, and mitochondrial deformation with no significant abnormal deposits of glycogen according to electron microscope analysis (Fig. 2). She developed pulmonary congestion and frequent ventricular tachycardia, which were unsuitable for medical treatment. The patient therefore underwent surgical atrial septal defect (ASD) and ventricular septal defect (VSD) closure at 5 months of age. However, her HCM failed to improve after surgery and she developed chylothorax and chalodropsy, which were refractory and resistant to thoracentesis, mechanical ventilation, and intrapleural talc treatment. The patient finally died of chylothorax, malnutrition, anasarca, and cardiac failure at 11 months of age. An autopsy and PTPN11 mutation analysis of her parents were refused.
DISCUSSION
The overall frequency of HCM is estimated to be about 0.47/100,000 children. HCM is associated with a wide range of disorders, including familial HCM, metabolic disorders, neuromuscular disease, and syndromic HCM (NS, Costello syndrome, LEOPARD syndrome, cardio-facio-cutaneous syndrome) [Colan, 2010]. NS is a common syndromic cause of congenital heart disease, and a broad spectrum of congenital heart diseases have been reported in 50–60% of NS patients, including pulmonary valve stenosis, ASD, VSD, mitral valve dysplasia, coarctation, and HCM [Prendiville et al., 2014]. HCM occurs in about 20% of patients with NS. Although it does not affect the need for surgery, it has a significant impact on long-term survival [Hickey et al., 2011].
The frequency of HCM increases to 85–90% in patients with NS and LEOPARD syndrome with RAF1 mutations [Pandit et al., 2007]. RAF1 is activated by GTP-bound RAS and phosphorylates serine residues of MEK1 and MEK2. Gain-of-function RAF1 mutations appear to be causally associated with the conserved region 2 (CR2) domain, and play a major role in cardiomyocyte hypertrophy. RAF1 mutations have two allelic specificities, and HCM is usually associated with mutations clustering around Ser259 and Ser612; however, these mutations were absent in the current case.
PTPN11 mutations are also frequently detected in NS and are positively associated with pulmonary stenosis, ASD, and HCM [Prendiville et al., 2014]. Gain-of-function mutations in RAS-MAPK signaling pathway genes occur in about 60% of NS patients and affect the processes of morphological determination, organogenesis, and growth [Roberts et al., 2013]. PTPN11 encodes protein tyrosine phosphatase SHP2, which plays a major role in activating the RAS-MAPK pathway. The occurrence of the T73I mutation in our patient was significant because of its frequent occurrence in juvenile myelomonocytic leukemia and NS/MPD [Kratz et al., 2005]. T73 is located at the N-terminus of the SH2 domain in PTPN11, and the T73I mutation generates an SHP2 gain-of-function mutation with a 6.3-fold increase in relative phosphatase activity [Niihori et al., 2005]. To the best of our knowledge, this is the first report of a T73I mutation in an NS patient with HCM. Interestingly, there is a clear association between HCM and PTPN11 mutations in exons 7 (Tyr279), 12 (Thr468), and 13 (Gln510) [Sarkozy et al., 2005]. These mutations are frequently found in patients with LEOPARD syndrome (also termed NS with multiple lentigines). Faienza et al. [2009] reported a newborn with NS phenotype and lethal progressive HCM who carried a loss-of-function mutation (Gln510Glu) in PTPN11. Gln510Glu caused HCM in mice by dysregulating mTOR signaling.
There are three possible explanations for the effect of the PTPN11 T73I mutation on HCM development. First, another, non-syndromic HCM-related gene mutation may also have been present in this patient. HCM is primarily a sarcomere disease involving the proteins that affect cardiomyocyte actin–myosin coupling. Over 400 mutations in about 13 sarcomere-related genes have been reported, and myosin, tropomyosin, troponin, myosin-binding protein C, and actin-related genes may all affect HCM [Lind et al., 2006]. Second, this patient may have displayed somatic mosaicism, especially in cardiomyocytes. To date, next-generation sequencing has revealed several somatic mosaicisms. Docker et al. [2014] reported a male patient with a coexisting germline PTPN11 mutation and a somatic PIK3CA variant, suggesting that indeterminate somatic variants may exist that could explain the co-occurrence of symptoms. Third, some other pathway related to the enhanced phosphorylation of mutant SHP2 may trigger the development of HCM. Recent research suggests that some phosphorylated SHP2 is transferred to the nucleus and affects other pathways, such as telomerase reverse transcriptase (TERT), signal transducer and activator of transcription 1 (STAT1), or Wnt signaling [Takahashi et al., 2011], which in turn may affect cell proliferation, morphological determination, organogenesis, and growth.
In conclusion, we observed a Japanese infant with NS and a T73I mutation in PTPN11 who displayed NS/MPD, CAVSD, and rapidly progressive HCM. This patient provided us with more information about the genotype–phenotype correlation between PTPN11 mutation and pathological characteristics such as HCM. Further evidence from phenotypically or genetically confirmed patients will help to clarify the underlying pathophysiology of NS.