Atypical Williams syndrome in an infant with complete atrioventricular canal defect
Abstract
Williams-Beuren Syndrome (WBS) is a well-described microdeletion syndrome characterized by specific dysmorphic facial features, peripheral pulmonic stenosis, supravalvular aortic stenosis, hypercalcemia, feeding difficulties, gastroesophageal reflux, short stature, and specific intellectual disabilities (such as visual spatial problems). WBS is caused by 7q11.23 deletions that contain multiple genes known to contribute to the above phenotype. We report a neonate with a complete atrioventricular canal (CAVC) defect, an atypical cardiac lesion for WBS, and few typical phenotypic features of WBS, diagnosed at 20 days of life. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Williams-Beuren Syndrome (WBS) is a multi-systemic disorder caused by heterozygous microdeletions of 7q11.23. Typical deletions range in size from 1.4 to 1.8 million base pairs and contain 26–28 genes [Kaplan et al., 2001; Pober, 2010]. Patients with WBS can demonstrate characteristic facial features, short stature, failure to thrive, endocrine disturbances, intellectual disability, and cardiac disease including vascular stenoses (Table I).
| Features | Previous case Nakamoto et al. [2003] | Present case, deceased at 3 months | Classic Williams syndrome (percentage of patients when available) |
|---|---|---|---|
| Facial features in early life: flat nasal bridge, upturned nose, periorbital fullness, small chin, stellate irides | + | − | + |
| Growth failure | + | − | + |
| (Percentiles at birth: | |||
| Weight: 50th | |||
| Height: 47th | |||
| Head Circumference: 85th) | |||
| Cardiovascular disease/Vascular stenosis | + | + | + |
| (80%) Pober et al. [2010] | |||
| Endocrine disturbances: hypercalcemia, hypothyroidism | Unknown | − | + |
| (Normal thyroid hormone and calcium levels) | (Hypercalcemia 15–50% Hypothyroidism 15–30%) | ||
| Pober [2010] | |||
| Renal anomalies | Unknown | − | + |
| (18%) Pankau et al. [1996] | |||
| Behavioral issues/Intellectual disability (often mild-moderate) | + | Unknown | + |
| (Intellectual disability 75% Behavioral problems 80%) | |||
| Mervis and Klein-Tasman [2000] | |||
| Hypotonia | Unknown | Unknown | + |
| Joint laxity | Unknown | − | + |
Descriptions of patients with WBS were initially published in the mid-20th century [Schlesinger et al., 1956; Bongiovanni et al., 1957; Williams et al., 1961; Beuren et al., 1962; Garcia et al., 1964]. More recently, the genetic etiology of WBS, specifically, heterozygous deletions of 7q11.23, was discovered through linkage analysis [Ewart et al., 1993]. ELN, which encodes elastin, was the first identified deleted gene and deficiency of this protein likely contributes to the arteriopathy in WBS patients. Elastin is an essential component of the extracellular matrix of arteries and is required for normal vascular wall development. Disruption of elastin function can induce subendothelial smooth muscle proliferation leading to narrowing of vessel lumens [Li et al., 1998]. The exact mechanism through which loss of ELN leads to arteriopathy, such as supravalvular aortic stenosis (SVAS), is not known. Williams-associated vascular disease demonstrates variable expressivity and reduced penetrance suggesting that other genetic modifiers influence the phenotype [Merla et al., 2012].
The complex WBS phenotype is likely due to the heterozygous deletion of many genes located in the WBS critical region. Through mouse models and studies of patients with atypical (usually smaller) deletions, attempts have been made to elucidate the exact contribution of each gene (for a review see [Schubert, 2009]). More research is still required to definitely establish the relationship between individual deleted genes and the specific symptoms seen in WBS patients.
While the phenotype of WBS is variable and multisystemic, much of the morbidity and mortality of the disorder can be attributed to cardiovascular involvement [Collins, 2013]. Cardiovascular manifestations of WBS are very common, occurring in approximately 80% of WBS patients [Pober et al., 2008]. Ninety-three percent of patients diagnosed in the first year of life have cardiovascular disease, although they are often not appreciated prenatally. This cardiovascular system involvement results in a 25–100-fold increase risk of sudden cardiac death in WBS patients [Bird et al., 1996; Wessel et al., 2004].
Arterial stenoses are the most common cardiovascular anomalies in WBS patients. SVAS is present in 45–75% of patients, and patients often have accompanying aortic valve abnormalities. Peripheral pulmonary arterial stenosis is present in approximately 40–65% of patients and tends to improve over time. Arterial stenosis also occurs fairly commonly in the aortic arch (coarctation) and less often in the descending aorta and renal arteries [Collins et al., 2010a,2010b; Collins, 2013]. Patients frequently have more than one vascular abnormality. In a study of cardiac catheterization in 242 WBS patients, 58% had SVAS associated with either pulmonary arterial stenosis or coarctation of the aorta [Pham et al., 2009]. Coronary artery stenosis is also seen in patients with WBS and is associated with an increased risk of adverse outcomes including sudden cardiac death [Bird et al., 1996].
While less common, structural heart disease can also occur in WBS. Ventricular septal defects (VSDs) are present in 4–13% of patients, the majority of which are muscular-type. Complete atrioventricular canal (CAVC) defects are not typically seen in WBS [Collins et al., 2010a,2010b; Collins, 2013]. There has been one reported case of CAVC in a living patient with WBS [Nakamoto et al., 2003].
We report an infant diagnosed prenatally with a CAVC defect and found to have WBS on postnatal testing. This case is unique in that the patient presented with a very atypical heart defect and lacked many other characteristic features of WBS.
CLINICAL REPORT
The patient's mother, a 22-year-old G2P0 woman, presented at 35 weeks and 2 days gestation for a prenatal genetics evaluation following identification of a CAVC on prenatal ultrasound. The pregnancy had been otherwise unremarkable. There was no known family history of congenital anomalies or heart disease; however, neither parent has had an echocardiogram. Reproductive history was notable for a prior first trimester miscarriage of unknown etiology. Cell-free non-invasive prenatal testing at her referring institution showed low-risk for Trisomy 13, 18, and 21 and showed the presence of a Y chromosome, suggesting a male fetus. A fetal echocardiogram and ultrasound were done at 35 weeks gestation because of concern for congenital heart disease on a routine anatomy ultrasound. The studies confirmed the presence of a CAVC, mild right ventricular hypertrophy, and a narrow, tortuous aortic arch. Otherwise, the anatomy was unremarkable and growth was between the 25th and 50th percentiles for fetal age. The mother declined additional prenatal genetic testing. Follow-up ultrasound performed at 36 weeks 4 days showed no worsening of cardiac function but lack of interval fetal growth since evaluation 9 days prior.
Labor was induced at 36 weeks and 6 days; cesarean section was necessary because of worsening fetal distress. The baby weighed 3,070 g at birth. APGAR scores were 1, 2, and 8 at 5, 8, and 10 min, respectively. He required chest compressions and intubation in the delivery room.
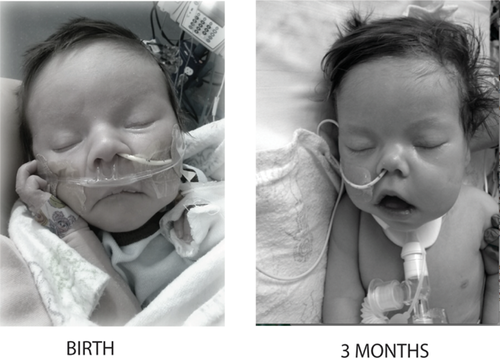
Physical examination at birth was notable for normal growth parameters (Table I). A genetics consultation was obtained and on initial examination he was noted to be nondysmorphic. However, on repeat examination at three months of age his nose was noted to have a broad, upturned tip, the philtrum was long, his cheeks were “full/jowly,” and his lips appeared fuller, “tented,” and with a decreased cupid's bow (Fig. 1).
Postnatal cardiac evaluation including echocardiogram confirmed the CAVC and showed a large muscular ventricular septal defect, interrupted aortic arch, and poor biventricular function. Chest radiograph demonstrated cardiomegaly and 13 pairs of ribs. On day of life nine (prior to WBS diagnosis), the patient had a cardiac procedure via catheterization with placement of a ductal stent and bilateral pulmonary artery bands. Post procedure, his significant cardiac dysfunction continued and he was listed for cardiac transplant.
In order to evaluate for subtle aneuploidy as an underlying etiology for his features, a genome-wide single nucleotide polymorphism (SNP) microarray was performed on blood. A 1.42 Mb deletion of 7q11.23 [arr 7q11.23 (72,722,981-74,141,840) x1] was detected. This deletion is consistent with the typical WBS recurrent deletion. This finding was confirmed by fluorescent in situ hybridization (FISH). Close scrutiny of the microarray showed no other abnormalities.
Given the diagnosis of WBS, magnetic resonance angiography of the head, neck, heart, chest, abdomen and pelvis was obtained to screen for vascular narrowing and tortuosity. Findings included an interrupted aortic arch and mild stenosis of the distal branch pulmonary arteries, the ascending aorta, the infra-renal abdominal aorta and the iliac arteries. No other vessel abnormalities were noted.
After the WBS diagnosis was discovered, the family was counseled appropriately and decided to pursue surgical correction of his congenital heart disease. He required tracheostomy, became more cyanotic and underwent a second catheterization procedure to augment his pulmonary blood flow at 3 months. In the setting of severe cardiovascular compromise, the parents withdrew care at three and a half months of age.
DISCUSSION
Williams Syndrome is a well-described microdeletion syndrome due to heterozygous deletions of 7q11.23. The diagnosis of WBS can often be made on clinical examination, even in the newborn period [Kaplan et al., 2001], by identification of characteristic facial features and a constellation of findings including chronic irritability in infancy, gastro-esophageal reflux, vascular stenosis, hypertension, hypercalcemia, failure to thrive, and developmental delay.
The infant described here lacked many typical WBS features. However, he died at 3 months of age and several WBS features including SVAS, endocrine disturbances, and growth failure may not be evident until after infancy. Most notably, his cardiac defect was a CAVC, which is not typically seen in WBS. There is one previous report of a WBS patient with a CAVC. Unlike our patient, this previous patient had several classic features of WBS including significant supravalvar aortic stenosis (SVAS), characteristic facial features, and growth failure (Table I).
The patient's atypical presentation delayed the genetic diagnosis until after he had his first surgical procedure. If this diagnosis had been known earlier it might have allowed the family and clinical teams to make better-informed decisions regarding his repair. Specifically, further vascular imaging would have likely been completed prior to his first surgery.
After the diagnosis of WBS was identified, the patient's cardiac function continued to worsen. The decision was made to list him for cardiac transplantation. At that time, comprehensive vascular imaging was obtained as part of his transplantation evaluation. Imaging revealed mild stenosis of several vessels, which is a common finding in WBS. However, the narrowing was not significant enough to prevent him from becoming a transplant candidate. His heart function worsened and he died awaiting transplant.
One limitation of this report is that we were unable to perform further testing, such as whole exome sequencing, to definitively exclude a second etiology of his congenital heart disease. However, he did not clearly have features of another identifiable syndrome.
The majority of CAVC defects are associated with genetic syndromes. This defect occurs most commonly in patients with Trisomy 21. In one series, 55% of patients with a CAVC defect were diagnosed with Trisomy 21. 14% of patients with CAVC were non-syndromic; while, the remaining 31% of patients had other genetic abnormalities [Carmi et al., 1992]. Other genetic causes of CAVC include chromosomal imbalances such as deletion 8p syndrome and several Mendelian disorders including Smith-Lemli-Opitz, Noonan, Ellis-van Creveld, and CHARGE syndromes [Digilio et al., 1999]. Animal models have implicated other genes that may contribute to the generation of CAVC. Specifically, loss of Wnt2 signaling in mice produces a CAVC-like phenotype [Tian et al., 2010; Gillers et al., 2015].
Here we present a rare case of a WBS patient with a CAVC defect. The occurrence of CAVC in at least two patients with WBS suggests that the lesion may be more common than previously thought in this patient population. Even though WBS was not initially suspected based on clinical examination in our patient, his diagnosis was ultimately obtained through SNP microarray analysis. Given the high prevalence of genetic disorders in association with CAVC defects, it is essential to pursue genetic testing beyond routine karyotype to identify chromosomal copy number variants in this population.