Brain MRI abnormalities and spectrum of neurological and clinical findings in three patients with proximal 16p11.2 microduplication
Abstract
The phenotype of recurrent ∼600 kb microdeletion and microduplication on proximal 16p11.2 is characterized by a spectrum of neurodevelopmental impairments including developmental delay and intellectual disability, epilepsy, autism and psychiatric disorders which are all subject to incomplete penetrance and variable expressivity. A variety of brain MRI abnormalities were reported in patients with 16p11.2 rearrangements, but no systematic correlation has been studied among patients with similar brain anomalies, their neurodevelopmental and clinical phenotypes. We present three patients with the proximal 16p11.2 microduplication exhibiting significant developmental delay, anxiety disorder and other variable clinical features. Our patients have abnormal brain MRI findings of cerebral T2 hyperintense foci (3/3) and ventriculomegaly (2/3). The neuroradiological or neurological findings in two cases prompted an extensive diagnostic work-up. One patient has exhibited neurological regression and progressive vision impairment and was diagnosed with juvenile neuronal ceroid-lipofuscinosis. We compare the clinical course and phenotype of these patients in regard to the clinical significance of the cerebral lesions and the need for MRI surveillance. We conclude that in all three patients the lesions were not progressive, did not show any sign of malignant transformation and could not be correlated to specific clinical features. We discuss potential etiologic mechanisms that may include overexpression of genes within the duplicated region involved in control of cell proliferation and complex molecular mechanisms such as the MAPK/ERK pathway. Systematic studies in larger cohorts are needed to confirm our observation and to establish the prevalence and clinical significance of these neuroanatomical abnormalities in patients with 16p11.2 duplications. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
The phenotype of recurrent ∼600 kb microdeletion and microduplication on 16p11.2 is characterized by a spectrum of neurodevelopmental impairments including developmental delay (DD) and intellectual disability (ID), epilepsy, autism and psychiatric disorders; all are subject to incomplete penetrance and variable expressivity [Kumar et al., 2008; Weiss et al., 2008; Bijlsma et al., 2009; McCarthy et al., 2009; Shimojima et al., 2009; Fernandez et al., 2010; Shinawi et al., 2010; Zufferey et al., 2012]. The prevalence of either the microdeletion or microduplication has been estimated to be approximately 1% in individuals with autism and/or developmental delay [Weiss et al., 2008]. Rosenfeld et al. [2010] showed speech delays and behavioral problems to be the predominant features in 32 individuals with 16p11.2 microduplications who were referred for chromosomal microarray analysis because of developmental delay though these features were not as consistent as in individuals with microdeletions. Reciprocal alterations in gene dosage at 16p11.2 result in mirror and contrasting phenotypes [Shinawi et al., 2010]. Abnormal head size—macrocephaly in individuals with 16p11.2 microdeletion and microcephaly in individuals with the reciprocal microduplication—has been reported. More recently, an association of obesity with the deletion and significantly reduced postnatal weight and body mass index (BMI) among duplication carriers have been observed [Walters et al., 2010; Jacquemont et al., 2011].
A wide spectrum of minor and major congenital anomalies has been reported in patients with 16p11.2 genomic rearrangements. However, these anomalies do not appear to have a consistent pattern. They were reported in 50% of patients with duplications [Shinawi et al., 2010]. Rosenfeld et al. [2010] found congenital anomalies in 5 out of 32 patients with 16p11.2 microduplication, but clinical information was only complete for 10 patients. Malformations were also reported in 29/101 patients with 16p11.2 microduplications ascertained for ID/DD; 13 of the 29 patients had central nervous system anomalies [Jacquemont et al., 2011]. However, the precise prevalence of brain anomalies is difficult to assess because of ascertainment bias, as imaging studies were not systematically performed in all carriers of 16p11.2 microduplications. Nevertheless, abnormal central nervous system (CNS) imaging findings reported by now in patients with 16p11.2 microduplications included white matter changes and atrophy and gliosis of thalami [Shinawi et al., 2010], cerebellar anomalies including cysts, hypoplasia and abnormal striations, cortical dysplasia, myelination delay [Jacquemont et al., 2011] and syringomyelia [Schaaf et al., 2011]. Until now, no systematic correlation has been made between patients with similar brain anomalies and their neurodevelopmental and physical phenotypes.
In this case series, we describe the neurological course and brain MRI findings in three patients with proximal 16p11.2 microduplication including an unexpected neurological deterioration in one patient who was ultimately diagnosed with neuronal juvenile ceroid-lipofuscinosis (JNCL). We discuss the brain imaging abnormalities including ventriculomegaly and multiple T2 hyperintense lesions and suggest a potential mechanism for their formation. The clinical relevance of the neuroimaging findings is unclear and raises questions regarding the utility of routine brain MRI in the clinical care and surveillance of patients with 16p11.2 rearrangements.
PATIENTS AND METHODS
Ethics
Ethical approval was obtained from the University of British Columbia Clinical Research Ethics Board (Patient 1; H09–01228). Patients 2 and 3 were recruited and photographs were acquired after informed consent for publication in the medical literature was obtained.
Patient 1
The 18-year-old male patient is the first child of healthy nonconsanguineous parents of Caucasian descent. His 16-year-old sister has an anxiety disorder, which improved with venlafaxine. His maternal aunt is described with learning disabilities and manic-depressive illness; her children are reported to have learning problems.
The patient was born after 39 weeks of uneventful pregnancy via Cesarean section for breech presentation. His birth weight was 2,855 g (−1.47 SDS), length was 50 cm (−0.7 SDS) and occipitofrontal circumference (OFC) was 34.5 cm (−0.5 SDS). The patient exhibited hypotonia and poor weight gain in the first 2 weeks of life. The patient had relative microcephaly with OFCs at just below −2 SDS at 3 years 9 months and −1.8 SDS centile afterwards. Camptodactyly of 5th fingers was first noted at the age of 7 years and progressed to involve all fingers at age 18. At age 15, the patient was found to have left sided thoracic scoliosis at 25°, which progressed to 43° at 17 years of age.
The patient presented with delayed or mildly delayed motor milestones with unsupported sitting at 10 months, crawling at 12 months and first walking at 18 months. He had persistent gait instability. The patient exhibited significant speech delay, moderate intellectual disability and behavioral anomalies such as a generalized anxiety disorder, manic, and self-injurious behaviors leading to a diagnosis of pervasive developmental delay. In addition, he had mood lability, paranoid ideation, markedly impaired communication, including incessant talking. The patient is currently treated with sertraline and quetiapine fumarate with minimal improvement.
The patient had epilepsy since the first year of life characterized by short focal or generalized tonic–clonic seizures, mostly associated with fever. Awake and sleep EEGs were normal. His seizures were successfully controlled with antiseizure medications, which were discontinued at the age of 8 years. On physical examination at 18 years of age he exhibited dysmorphic features (Fig. 1A,B; Table I) and had generalized hypotonia, marked camptodactyly of all fingers, and an unstable gait. Romberg sign was negative. There were no focal neurological findings.

| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Gender | M | F | F |
| Duplication type/size (kb) | Proximal/555 | Proximal/575 | Proximal/579 |
| Coordinates (hg19) | 29,622,757–30,177,999 | 29,580,611–30,155,315 | 29,620,689–30,199,683 |
| Inheritance | Maternal | Unknown (mother was negative) | De novo |
| Age at diagnosis (years) of 16p11.2 microduplication | 18 | 11 | 13 |
| Age at first diagnosis (years) of neuroradiological findings | 4 | 10 | 11 |
| Age at last assessment (years) | 18 | 12 | 17 |
| Growth | |||
| Height (SDS) | 0.3 | −0.6 | 1.9 |
| Weight (SDS) | 1.5 | −1 | −0.04 |
| BMI | 27.1 | 15.6 | 18.4 |
| Head circumference (SDS) | −0.15 | −3.2 | −0.4 |
| Developmental delay | Global DD, pervasive developmental disorder, hypotonia | Global DD, developmental regression at 10 years of age | Mild delay (early) |
| Intellectual disability | Moderate | Moderate (IQ 63) | Low normal intelligence (IQ 82) |
| Behavioral anomalies | Obsessive compulsive symptoms, self-injury, significant anxiety, mood lability, incessant and perseverative talking | Autism spectrum disorder, anxiety, ADHD | ADHD (mild), social anxiety |
| Epilepsy/EEG | Generalized tonic clonic and focal seizures in early childhood, no EEG anomalies | Yes, multiple EEG abnormalities | None |
| Physical anomalies | |||
| Dysmorphic features | Square shaped face, straight long nose, overhanging tip of nose, prominent alae nasi, short philtrum, small ears, hypoplastic lobules, short neck | Deep-set eyes, prominent ears, broad nasal bridge, bulbous nose, smooth philtrum, overbite, thin lips | Facial asymmetry, large and low-set ears, hypertelorism, high arched palate, micrognathia |
| Skin | Severe eczema | Acne | None |
| Skeleton | Progressive left thoracic scoliosis, 43 degrees at 17.5 years (last assessment) and significant lumbar lordosis | None | Pectus carinatum |
| Limbs | Short thumbs, very proximal insertion, slender hands with reduced flexion and extension creases, camptodactyly of all 5 fingers (progressive over the years), small feet, sandal gap, leg discrepancy 4 cm, planovalgus feet | 5th finger clinodactyly | Joint hypermobility |
| Other | Myopia | Precocious adrenarche, gradual vision deterioration with non-recordable rod and cone responses on ERG | Myopia |
His diagnostic workup was normal and included karyotype analysis, molecular testing for Prader Willi and Fragile-X-syndromes, acylcarnitine profile, plasma amino acids, urinary organic acids, urine mucopolysaccharides, lysosomal enzyme activities including hexosaminidase A, galactocerebrosidase, arylsulphatase A and B, very long chain fatty acids (VLCFAs), phytanic acid and isoelectric focusing of transferrin.
Patient 2
This Caucasian female patient is 12 years of age. Her parents and her 16-year-old sister are healthy. Two of her mother's nephews and nieces have attention deficit hyperactivity disorder (ADHD), and another niece has obsessive–compulsive disorder (OCD), ADHD, depression, and possible Asperger syndrome. The father's brother has epilepsy and spastic dysphonia, and a sister has epilepsy, depression, and ADHD.
The patient was born full-term via repeat Cesarean to a 34-year-old G4P1 → 2 mother after an uncomplicated pregnancy. Her birth weight was 3,570 g (0.03 SDS) and her birth length was 49.5 cm (−0.77 SDS). The patient was admitted to the neonatal intensive care unit after delivery and required oxygen supplementation by mask for a short period of time. She passed the newborn metabolic and hearing screens.
The proband was closely followed by endocrinology for benign premature adrenarche, which was first diagnosed at 2 years of age. She was followed by ophthalmology for stable myopia until she started exhibiting vision deterioration at 11 years of age. At age 12 an ophthalmologic examination revealed limited fixation, nystagmus and attenuation of the retinal blood vessels. An ERG showed non-recordable rod and cone responses.
The patient's formal IQ testing at age 7 years was 63 (Wechsler Intelligence Scale for Children, WISC). She was reevaluated at the age of 11 years for global DD, ID, epilepsy, ADHD, autism, and anxiety. The patient showed developmental regression in her motor skills over a year prior to her presentation. She has exhibited poor balance, unsteady gait, Parkinsonian features and progressive deterioration in her vision. Subsequent examinations over the next 1½ year revealed deterioration in vision with inability to visually track and nystagmus at mid position gaze. Her mild rigidity remained unchanged; however her gait continued to deteriorate to the point of requiring a walker, and mental status also declined with decreased ability to follow commands and loss of ability to identify colors, numbers, and letters.
On physical exam at 11 years of age (Table I), her weight was at −1 SDS, height was at −0.6 SDS and OFC at −3 SDS. There were some dysmorphic features (Fig. 1C,D; Table I). Her neurological examination initially showed marked motor apraxia, including limited ability to track objects with intact visual fields by confrontation. She had mild rigidity without spasticity, a wide based, shuffling gait with reduced arm swing and severe postural instability. Her mental status examination was consistent with a previous diagnosis of autism with limited eye contact, significant echolalia and perseveration.
Her diagnostic workup included normal CSF glucose, protein, cell count, lactate/pyruvate and aminoacids. Initial CSF neurotransmitter metabolites and 5-MTHF were normal, however repeat studies showed low homovanillic acid. Continuous video-EEG study obtained 1 year after initial evaluation was abnormal with drowsiness and sleep activated relatively rare bursts of irregular, generalized, 2–3 Hz spike and slow wave activity. The background activity during wakefulness lacked a well-developed anterior-to-posterior gradient and a posterior dominant rhythm, and stage II sleep was poorly developed. In addition, bursts of high voltage, generalized, semi-rhythmic to rhythmic notched delta activity were observed indicating mild to moderate generalized cerebral dysfunction of any etiology. All the following studies were normal: thyroid function, extensive metabolic and neurometabolic screening, fragile X syndrome, sequencing and deletion/duplications of MECP2, TCF4, SLC9A6, and UBE3A genes, and methylation for Angelman syndrome. Molecular testing of CLN3 revealed the classic homozygous deletion c.461–280_677+382del confirming the diagnosis of juvenile neuronal ceroid-lipofuscinosis (JNCL, Batten disease, OMIM204200).
Patient 3
This patient has been previously reported by Shinawi et al. [2010]. She was initially referred at 13 years of age for evaluation of “static encephalopathy” and DD. She was born after 38 weeks of uncomplicated pregnancy via spontaneous vaginal delivery. Her birth weight was 3,400 g (−0.3 SDS). Her neonatal period and infancy were uneventful except for left torticollis. The patient exhibited mild motor and speech delay and required therapeutic services. Her formal IQ testing yielded a score of 82. She was diagnosed with ADHD and social anxiety. She is usually rather solitary and does not participate in a lot of social events. There were no seizures, developmental regression or abnormal body movements. She had mild myopia but no other ophthalmological findings. Later on, the patient was noted to have some Marfanoid features including tall stature, pectus carinatum, and joint hypermobility, but she did not meet the clinical diagnostic criteria for Marfan syndrome. She wears orthodontic braces on the lateral aspects of her lower jaw to correct overbite caused by facial asymmetry.
On her physical exam at age 16 9/12 years there were dysmorphic features including facial asymmetry, large, low-set ears, hypertelorism, high palate, and micrognathia (Fig. 1E,F). She had pectus carinatum and joint hyperextensibility. Her neurologic examination was normal except for a slight reduction (10%) in vibratory sensation at the left great toe. Clinical signs are summarized in Table I.
Her diagnostic workup has included normal abdominal ultrasound and echocardiography. Her karyotype was normal.
RESULTS
MRI Findings
Representative MRI images are shown in Figures 2 and 3. The neuroradiological findings are summarized in the Table SI (see Supporting Information Online).
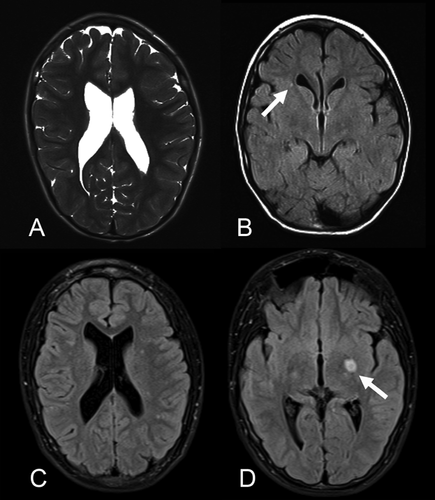
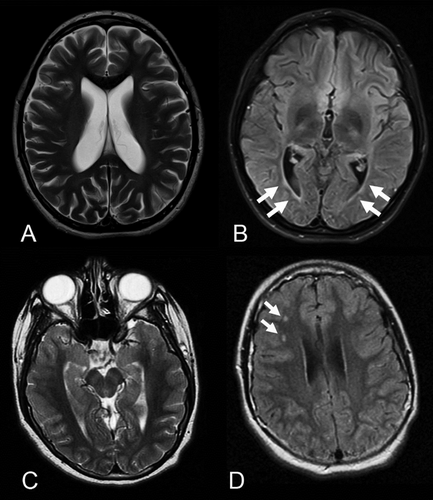
In Patient 1 the first head CT was done at 4 years of age and an enlargement of the third, fourth, and lateral ventricles without evidence for obstructive hydrocephalus was confirmed by MRI at 6 years 9 months. Those results were stable on all subsequent MRIs. At the age of 13 years, T2 hyperintensity was demonstrated in the left basal ganglia involving the globus pallidus and the putamen. In addition, multiple small T2 hyperintense foci were found adjacent to the trigones of lateral ventricles as well as in the more peripheral white matter of the frontal lobes and parietal lobes. Those smaller lesions were stable in follow-up MRIs. The larger lesion slightly increased in size and appeared with brighter signal at the age of 14 years, but less bright and stable in size since then. The last MRI follow-up was done at 16 years 9 months of age.
Brain MRI of Patient 2 at age 10½ years showed mildly enlarged dysmorphic posterior horns of both lateral ventricles and thinned periventricular white matter. The corpus callosum was mildly thinned in the posterior body and isthmus. Because of her progressive neurological course, repeat brain MRIs were performed after 6 and 18 months and showed stable enlargement of the ventricles and unchanged periventricular white matter thinning and gliosis with mild T2 hyperintensity.
Brain MRI of Patient 3 showed on two occasions stable foci with T2 hyperintensity in the right frontal white matter that were interpreted as foci of gliosis.
Chromosomal Microarray
Patient 1
Chromosomal microarray analysis (CMA) using CytoScan HD Array (Affymetrix, Santa Clara, CA) showed an approximately 555 kb copy number gain on 16p11.2 (chr16:29,622,757–30,177,999; hg19) corresponding to the proximal microduplication. Copy number analysis was done using the Chromosome Analysis Suite V1.2.2. Results were confirmed by fluorescence in-situ-hybridization (FISH) using BAC clones: RP11–3101D18, RP11–114A14 (TCAG Toronto). FISH analysis showed that patient's mother and sister are carriers for the same microduplication.
Patient 2
The CMA revealed a ∼575 kb-duplication on 16p11.2 (chr16:29,580,611–30,155,315; hg19). Microarray analysis was performed using Affymetrix Genome-Wide Human SNP Array 6.0 chip (Affymetrix) according to the manufacturer's protocol. The microduplication was confirmed by FISH using the RP11–74E23 clone (Empire Genomics) as a probe. Maternal FISH testing using the same probe was negative. The father declined testing. The classic homozygous 1.02 kb deletion c.461–280_677+382del in CLN3 (chr16p11.2:28396101–28411124; hg19) was inherited from both parents and is about 1 Mb telomeric from the microduplication. Careful re-examination of the array data did not show any evidence for a complex rearrangement including CLN3.
Patient 3
CMA using an Agilent customized HD-CGH microarray in 8 × 15K format (G4427A) to specifically interrogate 16p11.2 [Shinawi et al., 2010] revealed de novo 579 Kb microduplication on 16p11.2 (chr16:29,620,689–30,199,683; hg19). FISH analysis using the BAC clone RP11–301D18 was used to confirm the microarray results as previously reported.
DISCUSSION
16p11.2 rearrangements have been reported with variable phenotypes ranging from healthy carriers to severely disabled individuals presenting with a large spectrum of developmental and neurobehavioral abnormalities. The most common clinical findings among patients with the 16p11.2 duplication are developmental delay, especially in speech, and cognitive impairment [Fernandez et al., 2010; Rosenfeld et al., 2010; Shinawi et al., 2010]. Neurobehavioral problems such as ADHD and autism, schizophrenia, and seizures were also commonly associated with the 16p11.2 duplication [Kumar et al., 2008; Weiss et al., 2008; McCarthy et al., 2009; Shinawi et al., 2010]. Although dysmorphic features of patients with 16p11.2 duplication do not clearly constitute a clinically recognizable pattern, these are common and more severe compared to patients with the reciprocal deletion [Shinawi et al., 2010]. There is also increased incidence of congenital anomalies in individuals with the 16p11.2 duplication but with no predilection for a specific system [Schaaf et al., 2011].
Certain phenotypic features such as head size and BMI were systematically investigated and phenotype–genotype correlations were established. In a number of patients with 16p11.2 rearrangements, a variety of neuroradiological findings were reported, but until now, subgroups of brain anomalies have not been defined or correlated to the developmental phenotype. Interestingly, copy number changes of 16p11.2 in mice dictate gene expression levels in the brain and cause changes in the size and structure of distinct brain regions, where deletion and duplication exhibit opposing phenotypic effects [Horev et al., 2011].
Although microcephaly is well documented in patients with 16p11.2 duplications, other neuroradiological abnormalities have been rarely studied and reported. We present three patients who showed T2 hyperintense brain lesions in their MRI studies. In two patients (Patients 1 and 2), enlargement of brain ventricles was also seen. All patients show mild to moderate intellectual disability, anxiety disorder, some dysmorphic features, and other variable anomalies. The indication for MRI studies in all three patients was DD, combined with seizures in Patients 1 and 2. However, the clinical significance of the T2 hyperintense foci, which were interpreted as a type of gliosis, incomplete myelination or dysmyelination according to neuroradiological citeria, was unknown. In Patient 1, a few lesions were reminiscent of findings seen in neurocutaneous syndromes, but the patient did not exhibit skin findings compatible with neurofibromatosis type 1 (NF1) or tuberous sclerosis complex (TSC). Although there was suggestion of progression of the T2 hyperintense focus in the left basal ganglia in Patient 1, this could not be confirmed with further MRI studies. Overall, mild ventricle dilatation and T2 hyperintense foci have been stable in all three patients over time, and there were no signs for malignant degeneration. No biopsy was performed for any of the lesions because their locations were not amenable to surgical intervention or because non-invasive radiological surveillance was preferred in view of the apparent stable nature of these lesions. However, the significance of the MRI findings for patient surveillance and management, the risk of possible malignant transformation as well as their correlations to the patients' developmental phenotypes were unclear. These questions remained unsolved for each patient even after the diagnosis of the 16p11.2 microduplication was established because of hitherto lack of data on phenotype–genotype correlations.
It has been shown previously that transcription levels of MAPK3, YPEL3, CORO1A, and KCTD13 in lymphoblasts from patients with 16p11.2 rearrangements are positively correlated with 16p11.2 copy number [Luo et al., 2012]. Interestingly, the same study also showed 135 differentially expressed genes outside of the CNV region in 16p11.2-duplication cases suggesting trans regulation of multiple biological and molecular pathways [Luo et al., 2012]. Studies in zebrafish embryos confirmed that overexpression of KCTD13 induces the microcephaly phenotype associated with the 16p11.2 duplication, mediated through increased proliferation of neuronal progenitors in the developing brain [Golzio et al., 2012]. The duplicated 16p11.2 region encompasses 29 genes including a few interesting candidates for the neuroradiological endophenotypes in our patients (Fig. 4). These genes are involved in the control of cell proliferation and may explain or contribute to the mechanism of the cerebral lesions in our patients. TMEM291, which encodes the insulin-like growth factor binding protein-3 receptor (IGFBP-3R), is essential for IGFBP-3-induced apoptosis and tumor suppression as well as IGFBP-3 modulation of the balance between pro-apoptotic and pro-survival sphingolipids by regulating sphingosine kinase 1 and sphingomyelinases [Ingermann et al., 2010; Baxter, 2013]. Tao kinase 2 (TAOK2, OMIM 613199) is a serine/threonine kinase that has a role in cell signaling, microtubule organization and stability, and in apoptosis [Moore et al., 2000; Zihni et al., 2007]. The transcription factor myc-associated zinc finger protein, encoded by the gene MAZ (OMIM 600999), is expressed in human embryonic brain. It enhances the NMDA receptor subunit type 1 (OMIM 138249) activity during neuronal differentiation and was shown to play a role in cell cycle proliferation [Okamoto et al., 2002; Ray et al., 2004]. Another interesting candidate gene is the mitogen-activated protein kinase 3 (MAPK3 or ERK1; OMIM 601795), which encodes a protein that belongs to the MAP kinase family. MAP kinases, also known as extracellular signal-regulated kinases (ERKs), act in a signaling cascade that regulates various cellular processes such as proliferation, differentiation, and cell cycle progression in response to a variety of extracellular signals. Besides its implication in tumorigenesis, the RAS/MAP Kinase pathway plays a crucial role in Noonan and Noonan-related syndromes, now collectively called RASopathies [Rauen et al., 2010]. Neurofibromin, which is deficient in NF1 and activates RAS specific GTPase, is also implicated in this pathway [Tartaglia et al., 2011]. Cerebral hyperintense lesions such as neurofibromas, gliomas and hamartomas are well known in patients with neurocutaneous syndromes for which the disease mechanism has been linked to complex interactions in the RAS/MAPK, mTOR, and ERK signaling pathways [Kwiatkowski and Manning, 2005].

The nature of the T2 hyperintense foci in our patients remains unclear as biopsy and histologic examination was not performed. In neurologically healthy pediatric populations such foci occur in less than 1% [Kim et al., 2002], but are observed in up to 50% of pediatric patients with lupus as a result of vasoocclusive disease [Muscal et al., 2010]. Interestingly, in children with cognitive and or developmental delay white matter signal abnormalities are estimated to occur in 17–26% of cases. Such T2 hyperintense foci were thought to be rather non-specific findings, but recently they were associated with a deletion syndrome involving the TM4SF20 gene [Wiszniewski et al., 2013]. The authors suggested a toxic effect of the truncated protein as most likely mechanism. The T2 hyperintensities in the patients we describe appear radiographically similar to gliotic changes. We speculate that overexpression of genes within the 16p11.2 duplication involved in the regulation of cell proliferation may contribute to the development of cerebral gliosis in our patients.
Our clinical data suggest that the T2 hyperintense foci are most likely not correlated with a specific neurological or developmental outcome nor indicate disease progression. Particularly, the follow-up of two patients until age 17–18 years allowed this conclusion based on a long-term longitudinal observation. In Patients 1 and 2, a progression in some elements of their clinical phenotypes raised questions of potential relationship to the cerebral findings. Whereas in Patient 1 the aggravation of the anxiety disorder posed a significant challenge, Patient 2 had significant developmental regression associated with deterioration in her vision at the age of 10 years. The clinical findings in Patient 2 were ultimately explained by JNCL, which was confirmed by molecular testing. This observation suggests that additional diagnostic modalities should be considered in patients with 16p11.2 rearrangement who exhibit developmental regression and atypical presentation. Interestingly, CLN3 is also localized in the 16p11.2 chromosomal region, however, it is approximately 1Mb telomeric from the proximal 16p11.2 microduplication. Careful re-examination of the microarray data of our patient did not reveal additional 16p11.2 rearrangements as it was recently reported in a patient with JNCL with 16p11.2 microdeletion [Pebrel-Richard et al., 2013]. This proband was found to have a distal 200 kb microdeletion on 16p11.2, partially encompassing the CLN3 gene and unmasking a hemizygous 1.02 kb deletion mutation on the second allele. In our patient, the 16p11.2 microduplication and recessive inheritance of the homozygous CLN3 mutations seem to be independent events. Brain MRIs in patients with JNCL reveal cerebral, and to a lesser degree, cerebellar atrophy in later stages of the disease. T2 hyperintense foci are generally not reported, although thin perventricular, high-signal rims on T2-weighted images similar to the findings in Patient 2 were reported in one study [Vanhanen et al., 1995] (Fig. 3B). Patient 3 had an entirely stable clinical course, and she was 17 years of age at the last assessment.
In summary, we describe brain MRI findings and clinical course in three patients with 16p11.2 duplication and show that the T2 hyperintensities were stable and not associated with a specific clinical sign or disease progression. In the presence of developmental delay and the appearance of cerebral T2 hyperintense lesions, the 16p11.2 rearrangements should be considered in the differential diagnosis, and thus chromosomal microarray analysis is indicated in the initial diagnostic work-up. Additional studies are needed to determine the utility of brain MRI for assessment and surveillance of patients with 16p11.2 duplications and studies with larger cohort sizes and systematic neuroradiological assessments are needed to establish the precise prevalence of these anomalies in patients with 16p11.2 duplication.
The diagnosis of JNCL in one of our patients indicates that unexpected conditions should still be considered in patients with 16p11.2 rearrangements who show clinical progression or atypical neurological manifestations. The mechanism of the cerebral lesions is currently undetermined, but candidate genes regulating cell proliferation are likely contributory through complex signaling pathways.
ACKNOWLEDGMENTS
We are grateful to the patients and their families for their participation.