Enamel–Renal–Gingival syndrome, hypodontia, and a novel FAM20A mutation
TO THE EDITOR
Amelogenesis imperfecta, hypoplastic type (AI) with gingival fibromatosis (AIGFS; OMIM #614253) and amelogenesis imperfecta (AI) with nephrocalcinosis or enamel–renal syndrome (ERS; OMIM #204690) are rare autosomal recessive disorders which are caused by mutations in FAM20A [Cho et al., 2011; O'Sullivan et al., 2011; Jaureguiberry et al., 2013; Wang et al., 2013; Kantaputra et al., 2014; Wang et al., 2014]. A number of cases in the literature have described patients with three important findings including amelogenesis imperfecta, gingival fibromatosis, and nephrocalcinosis under the names amelogenesis imperfecta, hypoplastic type (AI) with gingival fibromatosis or ERS [Hall et al., 1995; Normand de la Tranchade et al., 2002; Paula et al., 2005; Kirzioglu et al., 2009; Jaureguiberry et al., 2013; Poornima et al., 2013; Wang et al., 2013]. Very recently, we have demonstrated that both disorders actually are the same entity as a number of previously reported patients with either syndrome sharing clinical findings, including AI, gingival fibromatosis, nephrocalcinosis, multiple unerupted teeth, heterotopic calcification in dental pulps, dental follicles and gingiva, and autosomal recessive inheritance. In addition, one of the reported patients also had nephrolithiasis (kidney stones) [Kantaputra et al., 2014]. The name “Enamel–Renal–Gingival syndrome” has been coined. The abnormalities found in patients with enamel–renal–gingival syndrome appear to be associated with biomineralization defects [Kantaputra et al., 2014]. Impaired calcium metabolism in a patient with amelogenesis imperfecta with nephrocalcinosis has been reported [Lubinsky et al., 1985]. Patients with homozygous FAM20A mutations and their heterozygous parents have been reported to have increased promotory activity and decreased inhibitory activity on calcium oxalate crystal growth [Kantaputra et al., 2014]. Physiologic calcification or biomineralization of extracellular matrices is a physiologic developmental process that is crucial for proper formation and functioning of various tissues, such as bones and teeth [Vogel et al., 2012]. FAM20A has been demonstrated to have inhibitory effects on biomineralization. That is why in the normal condition, we have mineralization in bones and teeth, not in gingiva, dental follicles, kidneys, and lungs. Mutations of FAM20A have been reported to be associated with mineralization of tissues, which in the normal situation are not mineralized. Here we report two additional patients affected with enamel–renal–gingival syndrome.
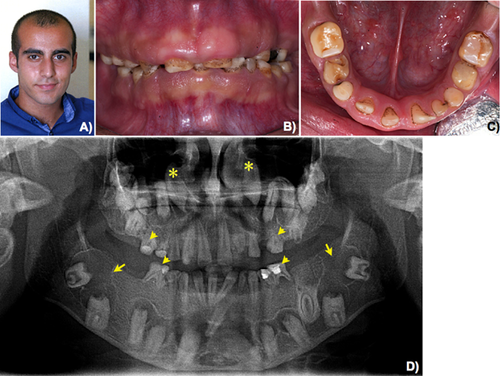
Patient 1 is a 17-year-old male of Turkish origin (Fig. 1A). He was the third child of nonconsanguineous parents. His parents had two spontaneous abortions. He presented for dental evaluation at The Department of Pedodontics, Faculty of Dentistry, Istanbul University, with amelogenesis imperfecta, hypoplastic type, and gingival fibromatosis. His health was otherwise healthy. Renal ultrasonography was normal. His serum calcium, phosphorus, and alkaline phosphatase levels were 10 mg/dl (normal 9.2–11.0), 3.6 mg/dl (normal 2.4–4.4), and 83 U/L (normal 75–390), respectively. Oral examination revealed amelogenesis imperfecta, hypoplastic type in his primary and permanent dentitions. Generalized gingival fibromatosis was observed (Fig. 1B,C). A panoramic radiograph showed generalized absence of enamel, prolonged retention of primary teeth, malposition of teeth, and multiple unerupted maxillary and mandibular permanent incisors, premolars, and molars. Very large dental follicles with sclerotic borders surrounded the unerupted teeth were evident. The dental follicles of the neighboring teeth appeared to connect to each other. The unerupted mandibular permanent second molars appeared to lie on the lower border of mandible. The unerupted maxillary permanent canines were high up to the level of the nose. Heterotopic calcification was observed in the dental pulps of the primary and permanent teeth (Fig. 1D). A diagnosis of enamel–renal–gingival syndrome was made.
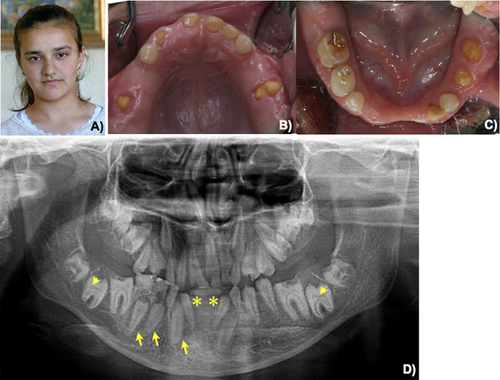
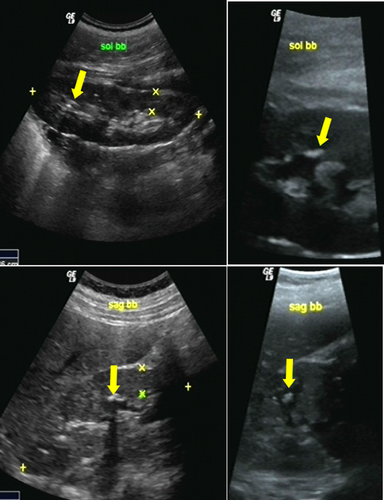
Patient 2 was a 14-year-old Turkish female (Fig. 2A). She was the second child of nonconsanguineous parents. Her older and younger brothers were normal. She came to the Department of Pedodontics, Faculty of Dentistry, Istanbul University for dental evaluation. Oral examination revealed amelogenesis imperfecta, hypoplastic type in her primary and permanent dentitions. Generalized gingival fibromatosis was observed (Fig. 2B,C). A panoramic radiograph showed generalized absence of enamel, prolonged retention of primary teeth, malposition of permanent teeth, hypodontia of the mandibular permanent central incisors, multiple unerupted maxillary and mandibular premolars, permanent incisors and molars. Heterotopic calcification was observed in the dental pulps of the primary and permanent teeth (Fig. 2D). The urinary creatinine and calcium levels were 120.2 mg/dl (normal 15–350) and 11.2 mg/dl (normal 0.5–35.7), respectively. Renal ultrasonography showed multiple coarse hyperechoic foci localized in the papilla and medulla of both kidneys, indicating nephrocalcinosis (Fig. 3A–D). A diagnosis of enamel–renal–gingival syndrome was made.
Informed consent and 2 ml of peripheral EDTA blood samples from the patients and the parents were obtained under protocols approved by the Human Experimentation Committee of the Istanbul Medical Faculty, Istanbul University. Genomic DNA was extracted from the blood of the patients and from their normal parents using a Quickgene 610L DNA extracting machine (FUJIFILM, Tokyo, Japan). Mutation analysis of FAM20A was performed as previously described [Kantaputra et al., 2014]. Sequencing data were analyzed using the Sequencher software (Gene Codes Corporation, Ann Arbor, MI) for the identification of the point mutations.
MUTATION ANALYSIS
Mutation analysis of FAM20A in Patient 1 revealed a homozygous novel c.34_35delCT mutation in exon 1. The mutation is predicted to cause frameshift at position 12 (p.Leu12AlafsX67), leading to a premature termination codon at 78. Both of his normal parents were heterozygous for the mutation (Supplemental eFig. 1S-A in Supporting Information Online). This mutation has been reported in another Turkish patient [Cho et al., 2011]. Mutation analysis of FAM20A in Patient 2 revealed a homozygous novel c.1482_1483insAC mutation in exon 11. The mutation is predicted to cause frameshift at position 495 (p.Leu495ThrfsX13), leading to a premature termination codon at 507. Both of her normal parents were heterozygous for the mutation (Supplemental eFig. 1S-B in supporting information online). Both mutations found in FAM20A mutation screening of both families were not encountered in 100 chromosomes of normal controls and they were also not present in gene variant public databases, including NCBI, 1000 genome, EXOME variant databases, dbSNP, or Human Gene Mutation Database (HGMD). This absence would indicate that both mutations do not represent neutral polymorphisms. In addition the c.34_35delCT mutation has been reported to be a disease-causing mutation in a Turkish patient [Cho et al., 2011]. Both molecular alterations were predicted to be disease-causing variants by mutation prediction software, including SIFT, Polyphen2, MutationTaster, and HANSA. These lines of evidence have indicated that both mutations found in our study are likely to be disease-causing mutations.
IMMUNOHISTOCHEMISTRY
CD1 mice were used. Day E0 was taken to be midnight prior to finding a vaginal plug. Embryo heads were fixed in 4% buffered paraformaldehyde, wax embedded, and serially sectioned at 7 µm. After deparaffinization of sections, sections treated by proteinase K and then incubated with antibody to FAM20A (sc-164310; Santa Cruz, Dallas, TX). Tyramide signal amplification system (NEL701001KT; Perkin Elmer Life Science, Waltham, MA) was performed for detecting the primary antibody. Our study has demonstrated that Fam20a protein was also observed in tooth germs at the very early stages of tooth development (Fig. 4A–D).
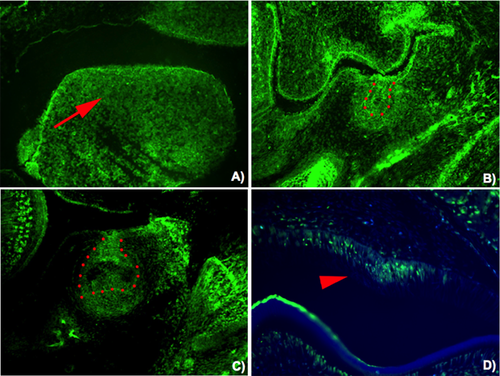
Hypodontia found in Patient 2, appears to be a common finding but has not been considered an important finding of the syndrome [Peters et al., 1992; Atasu et al., 1999; Raubenheimer and Noffke, 2002; Carnelio and Rao, 2005; Martelli-Junior et al., 2008; Cho et al., 2011]. Fam20a has been reported to be expressed by secretory but not maturation-stage ameloblasts, and this explains amelogenesis imperfecta, hypoplastic type in patients with FAM20A mutations [O'Sullivan et al., 2011; Wang et al., 2014]. Our study has demonstrated for the first time that Fam20a is expressed in tooth germs at the very early stages of tooth development (epithelial thickening, bud, and cap stages) and this supports hypodontia phenotype in a number of patients who had FAM20A mutations. Interestingly Fam20a expression in the dental follicles just above the molar cusp tips has been hypothesized to be involved in tooth eruption. Its absence has been postulated to be involved in failure of tooth eruption as commonly seen in patients with FAM20A mutations [Wang et al., 2014]. Our study reports a novel and a previously reported FAM20A mutations and confirms that ERS and amelogenesis imperfecta-gingival fibromatosis syndrome actually are the same entity with different manifestations, caused by mutations in FAM20A. Patients with amelogenesis imperfecta, hypoplastic type with unerupted teeth, and gingival fibromatosis are advocated to have renal ultrasonography to rule out nephrocalcinosis or nephrolithiasis.
ACKNOWLEDGMENTS
We thank our patients and their families for their kind cooperation and for allowing us to use their medical and dental information for the benefit of others. We are thankful to Dr. Kayoko Nozawa-Inoue for his assistance in immunohistochemical study. This research project was supported by Center of Excellence in Medical Genetics Research, Chiang Mai University and the Thailand Research Fund (TRF). The authors wish to thank Dr. M. Kevin O Carroll, Professor Emeritus of the University of Mississippi School of Dentistry, USA, and Faculty Consultant at Chiang Mai University Faculty of Dentistry, Thailand, for his assistance in the preparation of the manuscript.