Macrocerebellum, epilepsy, intellectual disability, and gut malrotation in a child with a 16q24.1–q24.2 contiguous gene deletion
Abstract
Macrocerebellum is a rare condition characterized by enlargement of the cerebellum with conservation of the overall shape and cytoarchitecture. Here, we report on a child with a distinctive constellation of clinical features including macrocerebellum, epilepsy, apparent intellectual disability, dysautonomia, gut malrotation, and poor gut motility. Oligonucleotide chromosome microarray analysis identified a 16q24.1–q24.2 deletion that included four OMIM genes (FBXO31, MAP1LC3B, JPH3, and SLC7A5). Review of prior studies describing individuals with similar or overlapping16q24.1–q24.2 deletions identified no other reports of macrocerebellum. These observations highlight a potential genetic cause of this rare disorder and raise the possibility that one or more gene(s) in the 16q24.1–q24.2 interval regulate cerebellar development. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Macrocerebellum is a rare condition characterized by enlargement of the cerebellum with conservation of overall shape and cytoarchitecture [Poretti et al., 2012]. Despite varied reports on syndromic and nonsyndromic cases of macrocerebellum, the underlying genetic etiologies remain largely unknown. We report on a child with macrocerebellum, epilepsy, cognitive impairment, dysautonomia, gut malrotation, and poor gut motility. We characterized the phenotype in this patient, evaluated her with array CGH, and compared her deletion and phenotype with other reports of patients with overlapping deletions.
CLINICAL REPORT
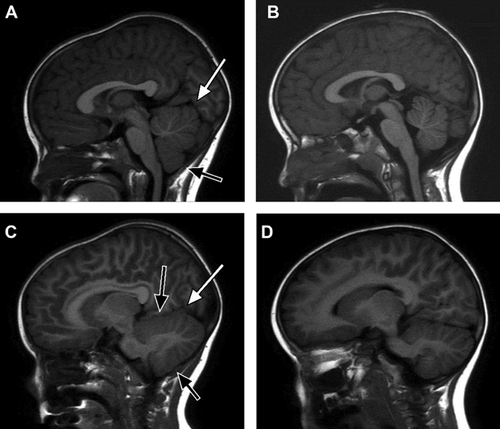
A 3-year-8-month-old girl was referred to the University of Michigan Pediatric Genetics service with a history of macrocerebellum, epilepsy, apparent intellectual disability, dysautonomia, gut malrotation, and poor gut motility. She was born at full term gestation after an uncomplicated pregnancy to a gravida 6 para 4 mother. Birth parameters included weight 3.4 kg (∼75th centile), length 51 cm (∼75th centile), and OFC 35 cm (∼75th centile). Initial concerns raised at 2–3 weeks of age included persistent screaming, prominent tongue thrusting, and hypotonia. She had been evaluated by the Beaumont Hospital Pediatric Neurology service at 2 months of age, when cranial MRI showed an enlarged cerebellum (Fig. 1). She underwent clinical genetic evaluation at Beaumont at 6 months of age, which included a genomic microarray analysis using Nimblegen CGX-3™ 135 K oligonucleotide array (v2.0 based on UCSC 2006 hg 18 assembly). This study showed a 983.10 kb deletion of chromosome 16q24.1–q24.2 (85,457,391–86,440,486; hg18). The proximal normal array feature is 85,454,268 and the distal normal array feature is 86,529,493. The array features for the proximal and distal deletion boundaries are 85,457,391 and 86,440,426, respectively.
Fluorescence in situ hybridization (FISH) analysis of metaphase cells using a BAC clone, which localized to the deleted region (RP11-178L8), confirmed the deletion. The FISH studies on 100 interphase nuclei from each parent showed no evidence of deletion, consistent with apparently de novo occurrence. The 16q24.1–q24.2 deletion resulted in loss of four OMIM genes: FBXO31, MAP1LC3B, JPH3, and SLC7A5 as well as three non-OMIM genes; C16orf95, ZCCHC14, and KLHDC4 (Fig. 2). The deletion was considered to be likely clinically significant based on the size of the deletion and its gene content.

Due to persistent discomfort and constipation, abdominal laparoscopic surgery was performed at seven months and showed a duodenal hernia and gut malrotation. She developed significantly reduced bowel motility and symptoms of autonomic dysregulation including decreased heart rate variability during wakefulness, lack of ventilatory and cardiovascular responses to mild daytime hypoxemia, acrocyanosis, intermittent fevers, and anisocoria. Normal enteric ganglion cells on biopsy ruled out Hirschsprung disease, and sequencing of the RET and PHOX2B genes was normal.
Developmental history was positive for mild global developmental delay. She rolled over by 5 months, crawled by 12 months, and walked by the age of 22 months. Shortly after turning 3 years old, she began using 2–3 word combinations and speaking in short sentences. Overall, she was noted to have significant difficulty with motor planning and word recall. She developed seizures at 15 months.
Due to the progression of abdominal distention, feeding intolerance, and hypotonia, a mitochondrial evaluation was pursued. A rectus abdominus muscle biopsy at age 3 years showed grouped atrophy of muscle fibers, suggestive of denervation. Carnitine was low (23.6 mmol/ml; normal 33.9–69.1) and free carnitine was 14.2 mmol/ml (normal 30.2–56.8). Creatine phosphokinase (CPK) was 326 U/L (normal 30–250). Electron transport chain analysis at the Center for Inherited Disorders of Energy Metabolism (Case Western Reserve University School of Medicine) indicated normal activity of ETC complex 3, but mild defects in complexes 1, 2, and 4. Mitochondrial DNA genome sequencing showed homoplasmy for a variant of unclear significance, m.11253T>C, p.Ile165Thr in the ND4 gene, which encodes a structural subunit of complex 4.
She had a history of mildly elevated lactate, pyruvate, and CPK; however, these were normal on repeat assessment at 3 years and 10 months of age. Additional biochemical evaluation at 3 years 10 months included plasma amino acids, urine organic acids, and carnitine combination profile, all of which were normal. Thymidine phosphorylase enzyme assay to evaluate for mitochondrial neurogastrointestinal encephalopathy (MNGIE) was normal. Testing for congenital disorders of glycosylation, including N-glycan and O-glycan screening, was normal. Sequence analysis of epilepsy-associated genes at 3 years 3 months (Courtagen Life Sciences, Woburn, MA) showed no pathogenic mutations in STIL, ATR, VPS13B, UBE3A, SLC25A33, CACNA1S, ACAD11, SIRT5, MUT, CFTR, DUSP26, LIPT2, METTL17, SPTLC2, EARS2, and ACO2 genes.
Physical examination at age 3 years 10 months showed weight 12.8 kg (∼5th centile), height 93 cm (∼5th centile), and OFC 50.2 cm (∼55th centile). Facial features were notable for bilateral mild epicanthal folds and a deeply grooved philtrum. Abdominal exam showed a gastrostomy tube with no surrounding erythema, swelling, or drainage. Neurologic exam demonstrated central hypotonia with normal strength in the extremities, wide based gait, hyporeflexia, and past-pointing on finger-nose examination. All other physical features were normal. A renal ultrasound performed at 3 years 10 months was normal.
Noncontrast cranial MRI at 3 years 7 months of age demonstrated diffuse symmetrical enlargement of the cerebellum with thickened but symmetric prominence of the gray matter most noticeable along the lateral and inferior aspects the cerebellar hemispheres (Fig. 1). The patient was imaged on a 3T Philips Ingenia MRI system using a 15-channel receive head coil. T1-weighted 3D-MPRAGE imaging was used to obtain the volumetric imaging data. Cerebellar volumetric analysis and segmentation was performed using FreeSurfer, version 5.3.0 (http://surfer.nmr.mgh.harvard.edu). The gray matter cerebellar volume was 94 cm3 and the white matter cerebellar volume was 31 cm3 for a total volume of 125 cm3. Based on normative imaging data, the volume of the cerebellum is equivalent to that of a 17–18 year old female [Brain Development Cooperative, 2012]. No underlying parenchymal signal abnormalities were identified. Cerebellar tonsils extended to the upper limit of normal at approximately 6 mm below the level of the foramen magnum and were morphologically normal. Supratentorial ventricles and cortical sulci were age-appropriate. No parenchymal signal abnormalities were identified and myelination pattern appeared normal. There were no midline shifts, mass effect, or hydrocephalus. Normal skull base arterial flow voids were present. No abnormal contrast uptake was seen. Magnetic Resonance spectroscopy performed over the basal ganglia and centrum semiovale showed no evidence of altered metabolite ratios. No lactate peak was noted.
DISCUSSION
A comparison of this child's features to those reported with 16q24.1–q24.2 deletions did not show any other reports of macrocerebellum, but included three individuals with similar deletions and macrocephaly [Butler et al., 2012; Handrigan et al., 2013]. This may reflect under-reporting of macrocerebellum as a radiologic observation, or modifying environmental or genetic factors.
Macrocerebellum has been reported as part of several genetic syndromes, including macrocephaly-capillary malformation syndrome [Conway et al., 2007a,b], Sotos syndrome [Schaefer et al., 1997], Williams syndrome [Jones et al., 2002], Costello syndrome [Gripp et al., 2010, 2011], Alexander disease [Torreman et al., 1993; van der Knaap et al., 2005], fucosidosis [Kau et al., 2011], and Lhermitte–Duclos syndrome [Milbouw et al., 1988; Nowak and Trost, 2002; Perez-Nunez et al., 2004]. In syndromic conditions that present with macrocerebellum, cerebellar growth often exhibits a distinct developmental pattern. Patients with Costello syndrome, macrocephaly-capillary malformation syndrome, and Lhermitte–Duclos syndrome all demonstrate gradual cerebellar enlargement. In patients with Alexander disease and fucosidosis where macrocerebellum has been reported, subsequent cerebellar atrophy was also identified [Inui et al., 2000; van der Knaap et al., 2005]. In the patient reported here, the overall proportion of cerebellum to cerebrum remained stable over time.
Macrocerebellum has been reported as an isolated occurrence in only ten individuals [Bodensteiner et al., 1997; Pichiecchio et al., 2011; Poretti et al., 2012]. These reports described additional clinical features that were not present in the child described here. Bodensteiner et al. [1997] described four patients with macrocerebellum and delayed myelination of the cerebral white matter, all of whom presented with global developmental delay, marked early hypotonia, and delayed maturation of the visual system manifested by apraxia of gaze and delayed maturation of fixing and following. Pichiecchio et al. [2011] reported the first longitudinal assessment of an individual with cerebellar enlargement, demonstrating increased cerebellar volume between birth and 19 months of age. They hypothesized that isolated enlargement of the cerebellum may not be a primary manifestation but rather an epiphenomenon secondary to disturbed cerebral involvement. Finially, Poretti et al. [2012] demonstrated thickening of the cortical gray matter of the cerebellar hemispheres and macrocerebellum in five patients. These individuals differed in dysmorphic features and extracerebral organ involvement, but all demonstrated hypotonia and delays in cognitive development. These varied features associated with macrocerebellum suggest broad clinical variability in expression and potentially substantial genetic heterogeneity; indeed, the child had a history of generalized weakness but no overt ataxia.
In the child described here, deletion of 983.1 kb of genomic material on chromosome 16q24.1q24.2 led to loss of one copy each of four OMIM genes and three non-OMIM genes (Table I). Handrigan et al. [2013] reported a series of patients with autism spectrum disorder, intellectual disability, congenital renal malformation, and a microdeletion within band 16q24.2. The present patient's deletion was not identical to any of those reported in their study; however, it did overlap on the centromeric side with their minimally deleted region [Handrigan et al., 2013]. This overlap included the genes FBXO31, MAP1LC3B, C16orf95, and ZCCHC14. Of interest, none of the patients they reported were reported to have macrocerebellum, although three individuals had macrocephaly. While this might suggest that macrocerebellum is associated with loss of JPH3 or SLC7A5, macrocerebellum may have been present in those patients but not detected and reported.
| Gene | Full name | Position (hg18) | OMIM # | Known associated diseases | Function | Mouse mutant phenotype | Cerebellar expression | References |
|---|---|---|---|---|---|---|---|---|
| FBXO31 | F-box protein 31 | 85,918,094–85,974,900 | 609102 | Cancer | Degradation via Skp1-Cul1-Fbox protein complex; cell cycle regulator | Impaired neuronal migration, morphogenesis, and dendritic growth in cerebellar/hippocampal neurons | Expressed in granule cells; expression in other cell types is unknown. | Kumar et al. [2005], Santra et al. [2009], and Vadhvani et al. [2013] |
| MAP1LC3B | Microtubule-associated protein 1 light chain 3 β | 85,983,302–85,995,881 | 609604 | None | Autophagy | None | Expressed, cell type unknown | Cann et al. [2008] |
| JPH3 | Junctophillin 3 | 86,192,142–86,289,262 | 605268 | Huntington's disease like 2 when gene has >41 CTG/CAG repeats | Endoplasmic reticulum/cell surface communication to mediate intracellular ion channels | Defects in motor coordination but cerebellar organization is intact | Purkinje cell layer of adult mice | Wilburn et al. [2011], Takeshima et al. [2000], Kakizawa et al. [2007], Nishi et al. [2002], and Nishi et al. [2003] |
| SLC7A5 | Solute carrier family 7 (amino acid transporter light chain, L system) member 5 | 86,421,130–86,460,601 | 600182 | Cancer | Neutral amino acid transporter | Embryonic lethal | Microvessels in Molecular layer, Purkinje cell layer, and white matter in adult rats | Kageyama et al. [2000], Tang et al. [2010], and Duelli et al. [2000] |
The International Standards for Cytogenomic Arrays Consortium (ISCA) database contains no deletions identical to that found in this study. Five smaller deletions that overlap the deleted region in this child are present in ISCA and are considered to be of unknown clinical significance. The ISCA database also documents two pathogenic deletions (2 and 9 Mb) associated with developmental delay, which overlap the deleted region reported here (including the distal portion of the deleted region). Also included in ISCA is a 1 Mb deletion (nearly identical to that reported here) considered of uncertain clinical significance but likely pathogenic. The phenotype listed for that case is macrocephaly. In none of these patients was macrocerebellum mentioned in the phenotypic description.
Recent data about the expression pattern and function of these genes provides evidence that haploinsufficiency of one or more of these genes may contribute to the macrocerebellum phenotype. FBXO31 is a member of the Fbox protein family, and regulates the cell cycle via the FBXO31–SCF (Skp1, Cullin1, Fbox protein) E3 ubiquitin ligase degradation pathway [Kumar et al., 2005; Santra et al., 2009]. The FBXO31–SCF complex regulates cultured neuronal morphogenesis and axonal outgrowth [Vadhvani et al., 2013]. Interestingly, RNAi knockdown of FBXO31 in rat cerebella impaired the ability of granule cell neurons to migrate out of the external granule layer [Vadhvani et al., 2013]. These observations suggest that FBXO31 may play a critical role in cell migration during cerebellar development.
MAP1LC3B, another gene in the deletion interval, encodes the microtubule associated protein 1 light chain 3 beta, a marker for induction of autophagy in tissues [Mizushima et al., 2004]. MAP1LC3B is highly expressed in the cerebellum [Cann et al., 2008]. Interestingly, autophagy and neurodegeneration are both implicated in seizure pathogenesis, and it is possible that haploinsufficiency for MAP1LC3B contributed directly to the cerebellar overgrowth and seizure phenotype in the patient reported here.
JPH3 (Junctophillin-3) encodes a member or the Junctophillin family of transmembrane proteins that span the endoplasmic reticulum and communicate with the cell surface through ion channels [Takeshima et al., 2000]. JPH3 and another family member, JPH4, are expressed throughout the brain in similar cell populations including cerebellar Purkinje cells [Nishi et al., 2003]. Neither JPH3 single mutant nor JPH3/JPH4 double mutant mice exhibit abnormalities in cerebellar structure; however, both mouse strains suffer from coordination and learning defects [Nishi et al., 2002; Kakizawa et al., 2007; Seixas et al., 2012]. Notably, CTG repeat expansions (>41) in the JPH3 gene cause Huntington disease-like 2 phenotypes [Wilburn et al., 2011].
SLC7A5 (also known as LAT1) resides in the deletion interval and encodes a neutral amino acid transporter expressed in microvessels and proliferating progenitors in the brain [Kageyama et al., 2000]. SLC7A5 is expressed in the hippocampus, subventricular zone, dentate gyrus, and cerebellum. Complete loss of Slc7a5 in mice is an embryonic lethal [Duelli et al., 2000; Kageyama et al., 2000; Tang et al., 2010]. SLC7A5 is also regulated in cortical tubers of patients with tuberous sclerosis and drug resistant epilepsy [Lim et al., 2011]. Thus, SLC7A5 may also contribute to cerebellar development and epilepsy. Other (non-OMIM) genes in the 16q24 deletion interval include ZCCHC14, KLHDC4, and C16ORF95, but their functions and expression patterns are unknown.
In summary, this child has a distinctive constellation of clinical features including macrocerebellum and a microdeletion of 16q24.1–q24.2. Further study of the expression and function of gene(s) in the deletion interval should shed light on the underlying etiology of macrocerebellum and provide clues about the genetic factors that regulate cerebellar growth and development.
ACKNOWLEDGMENTS
We thank David J. Aughton, MD for his participation in the care of this patient and for careful review of the manuscript. We also thank the family for their valuable input. D.M.M. is supported by the National Institutes of Health R01 DC009410 and University of Michigan Donita B. Sullivan MD Research Professorship Funds.