Characteristic brain magnetic resonance imaging pattern in patients with macrocephaly and PTEN mutations
Abstract
We describe an MRI phenotype seen in a series of patients with mutations in PTEN who have clinical features consistent with PTEN hamartoma tumor syndrome (PHTS). Retrospective review of clinical data and MRI was performed in 23 subjects evaluated in four different tertiary care centers with clinical programs in inherited disorders of the white matter. Patients were referred due to abnormal MRI features and abnormal PTEN sequencing was identified. All subjects had significant macrocephaly (on average >4 SD above the mean), developmental delay with or without autism spectrum disorder and uniform MRI features of enlarged perivascular spaces and multifocal periventricular white matter abnormalities. The phenotype of PHTS may include MRI abnormalities such as multifocal periventricular white matter abnormalities and enlarged perivascular spaces. These neuroimaging findings, in association with macrocephaly and developmental delay, should prompt consideration of PTEN as a diagnostic possibility. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
The PTEN hamartoma tumor syndrome (PHTS) refers to a group of clinical syndromes of aberrant growth due to germline mutations in the PTEN tumor suppressor gene [Blumenthal and Dennis, 2008]. There are a number of disorders associated with PTEN mutations, including Cowden syndrome, Bannayan–Riley–Ruvalcaba syndrome, and autism spectrum disorders with macrocephaly. Of note, Proteus syndrome (PS) recently correlated with AKT1 mutations in a majority of patients [Lindhurst et al., 2011], has in the past also been associated with PTEN mutations [Zhou et al., 2000; Smith et al., 2002; Hobert and Eng, 2009; Lindhurst et al., 2011]; however, it is unclear whether these patients have PS or a different condition.
Cowden syndrome (CS) is the phenotype most commonly associated with detectable PTEN mutations, reported in 80% of those with a clinical diagnosis of CS [Marsh et al., 1998]. It is characterized by mucocutaneous lesions (trichilemmomas, facial acral keratoses, papillomatous lesions), hamartomas, macrocephaly, and increased risk for breast, thyroid, and endometrical cancer [Blumenthal and Dennis, 2008; Pilarski, 2009]. Additionally, the presence of Lhermitte–Duclos disease, a cerebellar dysplastic gangliocytoma, is pathognomonic, but is also seen as an isolated manifestation of PHTS [Zhou et al., 2003; Chiofalo et al., 2007]. Under revised criteria, the majority of CS diagnoses are made in adulthood due to cutaneous findings, as malignancies are rarely present before 20 years of age.
Bannayan–Riley–Ruvalcaba Syndrome (BRRS), also known as Bannayan–Zonana Syndrome (BZS) is another clinical entity caused by PTEN mutations in 60% of cases [Marsh et al., 1998]. It is mainly characterized by developmental delay, macrocephaly, lipomas, hemangiomas, and pigmented speckled macules of the glans penis in males [Marsh et al., 1999; Blumenthal and Dennis, 2008]. This diagnosis is most often made in childhood.
Recently PTEN mutations have been identified in many children with macrocephaly associated with autism spectrum disorders and/or mental retardation as main symptoms [Butler et al., 2005; Lynch et al., 2009; Varga et al., 2009]. Estimated prevalence of macrocephaly (>2 SD above the mean) in patients with PTEN mutations ranges from 25 to 100% [Lachlan et al., 2007; Mester et al., 2011; Pilarski et al., 2011]. The variation in studies may reflect ascertainment bias, as early studies did not measure occipitofrontal circumference (OFC) and later studies tested for mutations predominantly in macrocephalic patients.
Neuroradiological findings in PTEN mutation positive patients are variable. In some patients neuroimaging is normal, while in others cortical and vascular malformations have been described [DeLone et al., 1999; Lok et al., 2005; Tan et al., 2007]. This report describes consistent brain MRI patterns in patients with macrocephaly (>2 SD above the mean) and developmental delay and/or autistic behaviors, which should lead to consideration of the PHTS.
METHODS
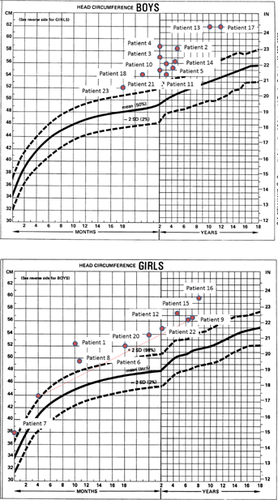
Patients were initially identified from a cohort of patients referred for unclassified white matter disorders who had characteristic clinical features of BRRS and abnormal PTEN sequencing (personal observation of MSvdK and CB). Subsequent patients were identified based on macrocephaly and/or developmental abnormalities in combination with brain MRI features and tested as part of routine clinical care using commercially available PTEN testing. Patients were collected from repositories of cases of unsolved white matter disorders with appropriate institutional review board approval, including the Myelin Disorders Bio-Registry Project (approved by the IRB of Children's National Medical Center for patients contributed by A.V. and A.W.), and by disease-specific repositories in Brazil (approved by the ethics board for patients contributed by C.L.), Italy (approved by the ethics board for patients contributed by S.O.), and the Netherlands (approved by the ethics regulatory board at the Vrije Universiteit Medical Center for patients contributed by MSvdK). MRIs and clinical histories were reviewed retrospectively for each patient. Clinical features including developmental delay, a diagnosis of autistism, and information on developmental outcome where available were noted. Head circumferences were plotted on a standardized graph to assess for macrocephaly [Nellhaus, 1968]. MRIs were reviewed for the presence of multifocal periventricular abnormalities of the white matter and enlarged perivascular spaces by A.V. Descriptive analysis only was performed in view of the small sample size.
RESULTS
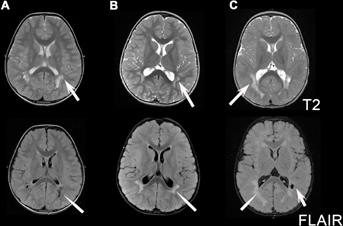
Twenty-three patients were collected with documented PTEN mutations and abnormal brain white matter on neuroimaging (13 males and 10 females). Patients presented for neurologic evaluation between the ages of newborn and 5 years (median 11 months, mean 1.6 years ± 1.5 years), in almost all cases for concerns related to macrocephaly and developmental delay. One patient (#12) presented for staring spells and concern for seizures. All patients had macrocephaly, with measurements available in all but one patient (Supplemental Data Table I—see supporting information online), defined as >2 SD above the mean related to age on a standard head circumference chart. Occipital frontal circumference measurements were well above means (mean of +4.5 SD above the mean) and medians (median of +4.3 SD above the mean) for age (Fig. 1). All patients also had developmental delay. Reports of delays were predominantly motor, as expected given the age at presentation, although six patients reportedly developed symptoms consistent with autism spectrum disorder. All patients had either periventricular abnormal signal of the white matter (n = 3) or enlarged perivascular spaces (n = 6), or both (n = 14) (Fig. 2). One case had isolated enlarged perivascular spaces, but the MRI was performed before 1-year of age limiting the interpretation of signal abnormalities within the deep white matter. All patients had documented mutations of PTEN, including missense mutations (n = 13), truncating mutations (n = 6), nonsense mutation (n = 1), deletion (n = 1), insertion (n = 1), and one other mutation resulting in the prolongation of the amino acid strand beyond the stop codon. One mutation was seen in two affected sisters and is counted once. Another patient had two potentially pathogenic mutations. Nearly half of the subjects (n = 10) had novel mutations. Two additional subjects had novel changes at amino acid sites with previously described mutations. When known, de novo versus familial occurrence was documented, but parental DNA testing was not available in all cases (in two cases neither parent was tested and in three cases only one parent was available for testing—Table I).
| Patient | Age at presentation (m = months y = years) | Gender | Macrocephaly (>>2SD above the mean for age) | Developmental delay | Physical exam | PTEN sequence analysis | Follow up | Posterior periventricular multifocal white matter abnormalities | Enlarged perivascular spaces with CSF isointense signal |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 m | F | + | + | Frontal bossing | c.388C>T; p.Arg130* [Nelen et al., 1997; Eng, 2003] | Clinical follow up at 3 years: pt was stable with gradual acquisition of milestones | + | + |
| Hypertelorism | De novo | ||||||||
| Hypotonia | |||||||||
| 2 | 5 m | M | + | + (Autistic features) | Frontal bossing | c.17A>T; p.Lys6Ile# mother unaffected, paternal status unknown | Clinical follow up at 5 years: pt was stable with gradual acquisition of milestones | + | + |
| Pigmented speckled macules of the glans penis | |||||||||
| Hypotonia | |||||||||
| 3 | 2 yr | M | + | + (Autistic features) | Frontal bossing | c.959T>G; p.Leu320* [Eng, 2003] parents not tested | Follow-up at 3 years: pt was stable with gradual acquisition of speech and improvement in socialization | + | + |
| Depressed nasal bridge | |||||||||
| Bulbous nose | |||||||||
| Smooth philtrum | |||||||||
| Mild hypotonia | |||||||||
| 4 | 1 y | M | + | + | Frontal bossing | c.320A>G; p.Asp107Gly# mother unaffected, paternal status unknown | Neuroradiologic and clinical follow-up at 2 y: pt was stable, with gradual acquisition of milestones | + | + |
| Hypertelorism | |||||||||
| Hypotonia | |||||||||
| 5 | 4.5 y | M | + | + (Autistic spectrum) | No dymorphisms | c.194A>G; p.Tyr65Cys# familial mutation | No clinical follow up | + | + |
| 6 | 11 m | F | + | + | Frontal bossing | c.853G>T; p.Gly285*, # | Persistent cognitive difficulties | None | + |
| Hypotonia | De novo | MRI stable over several years | |||||||
| Mucosal neuroma | |||||||||
| 7 | 11 m | F | + | + | Hypotonia | c.16A>G; p.Lys6Glu# parents not tested | Need for ongoing physical, occupational and speech therapy, stable MRI over several years | + | + |
| 8 | 10 m | F | + | + (Austic spectrum) | Frontal bossing | c.511C>G; p.Gln171Glu# familial mutation | Follow up at 3 y: limited expressive and receptive language | ++ | + |
| Hypotonia | |||||||||
| Left cataract | |||||||||
| 9 | 5 y (sister of 8) | F | + | + | None | c.511C>G; p.Gln171Glu# familial mutation | Follow up at 7 years: gross motor difficulties | None | + |
| 10 | 3 y | M | + | + | Frontal bossing | c.302T>C; p.Ile101Thr [Tan et al., 2011] | None | + | + |
| Hypotonia | De novo | ||||||||
| 11 | 3 y | M | + | + (Pervasive developmental disorder) | Frontal bossing | c.1211G>C; p.Stop404Ser# | Follow up at 5 years with gradual acquisition of milestones, in kindergarten with special education | None | + |
| Hypotonia | De novo | ||||||||
| 12 | 10 m | F | + | + | Hypotonia | c.80A>G; pTyr27Cys# | Follow up at 3 years with gradual acquisition of speech, in preschool | + | + |
| Abnormal EEG with epileptiform features but no seizures | De novo | ||||||||
| 13 | 3 y | M | + | + (Autistic spectrum) | Arteriovenous malformation of foot. No facial dysmorphism. | c.388C>T; p.Arg130* [Nelen et al., 1997; Eng, 2003] status unknown in mother, father not affected | Follow up at 16 y: autistic features | + | None |
| 14 | 5 y | M | + | + (Autistic spectrum) | Frontal bossing Down slanting palpebral fissures | c.737C>T; p.Pro246Leu [Eng, 2003] familial mutation | Acquiring new skills but requires special education | None | + |
| 15 | 3 m | F | + | + (Motor) | Hypotonia | c.1120_1121dup; p.Asp375fs# | Follow up at 32 months: verbal IQ 32 months, mild motor delay with level of 20 months | + | None |
| De novo | |||||||||
| 16 | 7 m | F | + | + | Frontal bossing Mild hypotonia | c.253 + 5G>T; p.? [Tan et al., 2011] | Follow up at 8 y: improved much, clumsy, follows normal education | + | + |
| De novo | |||||||||
| 17 | 18 m | M | + | + | None | c.45A>T; p.Arg15Ser (this exact mutation not found, but a mutation previously reported at the same amino acid [Tan et al., 2011] | None | + | None |
| De novo | |||||||||
| 18 | At birth | M | + | + (Mild motor only) | Macrosomia Frontal bossing Postaxial polydactyl | Partial deletion of exon 6, identified on array- CGH; arr 10q23.31 (89.683.610–89.702.204)x1 | Follow up at 2.5 y: hypotonia, mild motor retardation | + | + |
| De novo | |||||||||
| 19 | 10 m | M | + | + (Mild motor delay only) | Mild frontal bossing | c.131G>A; p.Gly44Asp [Varga et al., 2009; Rodriguez-Escudero et al., 2011] | None | + | + |
| Parents not tested | |||||||||
| 20 | 15 m | F | + | + | None | c.138C>G; p.Tyr46*# | None | + | + |
| Parents not tested | |||||||||
| 21 | 10 m | M | + | + (Autistic spectrum) | None | c.633C>G; p.Cys211Trp [Eng, 2003] | Follow up at 3 y: continued developmental gains; mild delay | None | + |
| Familial mutation | |||||||||
| 22 | 10 m | F | + | + (Motor delay) | Mild frontal bossing, split uvula, subcuta-neous lipomas abdomen, axillar trichilemmomas | c.1003C>T; p.Arg335* [Eng, 2003] | Follow up at 4 y: continued developmental gains | + | + |
| De novo | |||||||||
| 23 | 8 m | M | + | + | Macrosomia | c.149T>C; p.Ile50Thr (this mutation not reported, but mutation reported at this amino acid [Tan et al., 2011]) | None | <1 year at time of neuroimaging, cannot exclude | + |
| De novo |
- * (asterisk) = translation termination (stop) codon.
- # Cases with novel mutations, not previously identified in the literature but predicted to be pathogenic.
DISCUSSION
PHTS encompasses a group of clinical syndromes of aberrant growth due to germline mutations in the PTEN tumor suppressor gene. Recently, PTEN mutations have been identified in children with isolated macrocephaly and autism spectrum disorders and/or intellectual disability [Butler et al., 2005; Lynch et al., 2009; Varga et al., 2009]. These features, even in association, are non-specific, and the differential diagnosis is broad. For these reasons, the identification of a suggestive MRI pattern may help narrow diagnostic testing when pathognomonic clinical features (such as pigmented speckled macules of the glans penis) in childhood are not present. Prompt diagnosis may prevent unnecessary testing, such as genetic testing for leukodystrophies, and result in appropriate referrals to screening programs for hereditary cancers.
We report on a group of patients with genetically proven PTEN mutations with the presence of macrocephaly and developmental delay and/or autism spectrum disorders who have brain MRI abnormalities. On MRI, all patients had either static white matter multifocal abnormalities that were hyperintense on T2W and FLAIR images and hypointense on T1W images, and/or enlarged perivascular spaces. This pattern is not specific and is well known in other disorders including mucopolysaccharidoses [Seto et al., 2001; Kara et al., 2008], oculocerebrorenal syndrome of Lowe [Carvalho-Neto et al., 2009], and Hypomelanosis of Ito [Fryburg et al., 1996; Ruggieri et al., 1996]. We recognize that our subjects were selected for white matter abnormalities and it is unknown how frequent these findings are in patients with PTEN mutations. However, this MRI pattern in the appropriate clinical context should prompt consideration of the diagnosis of PTEN spectrum disorders.
The etiology of these white matter abnormalities is unknown, but they appear multifocal and static. In all 23 cases reported here, they were associated with the presence of dilated perivascular spaces, which have often been reported in small vessel brain diseases [Rouhl et al., 2008; Vahedi and Alamowitch, 2011]. It is of interest that in patients with known PTEN mutations a frequent finding is the presence of various vascular anomalies. Of interest Coffin–Lowry syndrome caused by mutations in RSK2, is also known to be associated with enlarged perivascular spaces in isolated cases [Kondoh et al., 1998].
In conclusion, the presence of multifocal static white matter abnormalities and dilated perivascular spaces should suggest the possibility of a PTEN spectrum disorder in a patient with macrocephaly and developmental delay and/or autism spectrum disorder. In patients with defined PTEN mutations or other signs or symptoms already known in this group of syndromes, multifocal white matter abnormalities and enlarged perivascular spaces can be considered to be part of the spectrum of this condition and should not prompt additional diagnostic testing.
ACKNOWLEDGMENTS
This work was supported by the Myelin Disorders Bioregistry Project. In addition, we acknowledge the support of the following physicians and practitioners: Carolina Funayama, MD, Miriam Bloom, MD of Children's National Medical Center, Washington DC, Dr. Giorgia Mandrile of the Department of Clinical and Biological Sciences, Orbassano, University of Turin, Italy, Bethany Friedman and Kathleen Hruska of GeneDx.




