Revisit of multiple epiphyseal dysplasia: Ethnic difference in genotypes and comparison of radiographic features linked to the COMP and MATN3 genes†
How to Cite this Article: Kim O-H, Park H, Seong M-W, Cho T-J, Nishimura G, Superti-Furga A, Unger S, Ikegawa S, Choi I H, Song H-R, Kim H W, Yoo WJ, Shim JS, Chung CY, Oh C-W, Jeong C, Song KS, Seo SG, Cho SI, Yeo IK, Kim SY, Park S, Park SS. 2011. Revisit of multiple epiphyseal dysplasia: Ethnic difference in genotypes and comparison of radiographic features linked to the COMP and MATN3 genes. Am J Med Genet Part A 155: 2669–2680.
Abstract
Multiple epiphyseal dysplasia (MED) is a genetically heterogeneous group of diseases characterized by variable degrees of epiphyseal abnormality primarily involving the hip and knee joints. The purpose of this study was to investigate the frequency of mutations in individuals with a clinical and radiographic diagnosis of MED and to test the hypothesis that characteristic radiological findings may be helpful in predicting the gene responsible. The radiographs of 74 Korean patients were evaluated by a panel of skeletal dysplasia experts. Six genes known to be associated with MED (COMP, MATN3, COL9A1, COL9A2, COL9A3, and DTDST) were screened by sequencing. Mutations were found in 55 of the 63 patients (87%). MATN3 mutations were found in 30 patients (55%), followed by COMP mutations in 23 (41%), and COL9A2 and DTDST mutations in one patient (2%) each. Comparisons of radiographic findings in patients with COMP and MATN3 mutations showed that albeit marked abnormalities in hip and knee joints were observed in both groups, the degree of involvement and the morphology of dysplastic epiphyses differed markedly. The contour of the pelvic acetabulum, the presence of metaphyseal vertical striations, and/or the brachydactyly of the hand were also found to be highly correlated with the genotypes. The study confirms that MATN3 and COMP are the genes most frequently responsible for MED and that subtle radiographic signs may give precious indications on which gene(s) should be prioritized for mutational screening in a given individual. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
The recognition of genetic heterogeneity in several skeletal dysplasias has substantially increased the number of genes which may need to be screened in order to confirm the clinical-radiographic diagnosis. Multiple epiphyseal dysplasia (MED) is the prime example of this as, to date, mutations in six different genes have been shown to cause MED; the genes encoding cartilage oligomeric matrix protein (COMP), matrilin-3 (MATN3), and the alpha 1–3 chains of type IX collagen (COL9A1, COL9A2, COL9A3), have all been shown to result in autosomal dominant MED, and a specific mutation in the diastrophic dysplasia sulfate transporter (DTDST) has been shown to be associated with an autosomal recessive form of MED [Rossi and Superti-Furga, 2001; Briggs and Chapman, 2002].
The frequencies of mutations in these genes in MED patients have yet to be clearly determined. Previous studies in European MED patients have shown a frequency of: 7–35% for COMP, 14% for DTDST, 5–10% for MATN3, and 5–15% for the type IX collagen genes [Briggs and Chapman, 2002; Jackson et al., 2004; Jakkula et al., 2005]. A comprehensive screening in a Japanese population indicated that frequencies differ in East Asian patients: 23% in MATN3, 20% in COMP, 6% in COL9A2, and none in DTDST [Itoh et al., 2006].
The purpose of this study was to determine molecular genetic characteristics in a cohort of clinically and radiographically defined MED patients. In addition, we also sought to identify radiological predictors of genotype, because mutation analysis of all six known genes in MED is a sizeable task and can be costly. We considered that this time-consuming procedure could be shortened by performing a preliminary assessment to target the molecular analysis. Thus, we hypothesized that knowledge of radiological phenotypes might be used to predict the causative gene, and that genotype-radiological phenotype correlation in MED might provide information that could guide gene analysis.
MATERIALS AND METHODS
Patients
Patients were selected first by pediatric orthopedists with over 10 years of experience at eight institutions. Clinical diagnoses were based on findings that included joint pain (particularly at hip and knee joints), a waddling gait, fatigue during walking, genu varum/valgum, and short stature. All patients received physical and radiographic examinations. Seventy-four patients were chosen by the pediatric orthopedists and were subsequently referred to the radiological review panel, which was composed of two pediatric radiologists (OHK and GN), and an expert pediatrician (ASF) and geneticist (SU) from the European Skeletal Dysplasia Network (ESDN). The panel performed a skeletal survey on each patient and then selected patients by consensus. Of the 74 subjects, 11 were excluded by the panel. In these subjects diagnostic hypothesis were: metaphyseal chondrodysplasia (four cases), pseudoachondroplasia (three cases), spondyloepiphyseal dysplasia (three cases), and spondyloepimetaphyseal dysplasia-leptodactyly type (one case). Sixty-three patients (aged from 2 to 28 years; 32 females and 31 males) were finally preselected by the panel for mutation screening.
Mutation Analysis
Approval for this study was obtained from the institutional review boards at each participating hospital. Genomic DNA was obtained from the circulating leukocytes of the 63 probands, and if possible, from their patients and/or siblings after obtaining informed consent. Gene screening was undertaken by directly sequencing exons 8-19 of COMP, exons 1 and 2 of MATN3, exons 8 and 9 of COL9A1, exons 3 and 4 of COL9A2, exon 3 of COL9A3, and all three exons of DTDST, and the flanking regions of all genes. Mutation analysis was firstly performed for COMP and expanded to MATN3, COL9A1, COL9A2, COL9A3, and DTDST in order. The following criteria were used to determine if novel sequence variations were pathogenic mutations; the chemical nature of the amino acids substituted, co-segregation with phenotype in a family, interspecies amino acid conservation, absence in at least 90 healthy individuals [Collins and Schwartz, 2002], and protein structural information predicted by Polyphen [Ramensky et al., 2002], SIFT [Ng and Henikoff, 2001], and PMut [Ferrer-Costa et al., 2004].
Radiologic Evaluations
Having completed the mutation analysis of the 63 patients, the radiographic findings of patients with confirmed mutations were re-evaluated for hips, knees, hands, ankles/feet, and the lateral spine. Hip and knee radiographs were available from all subjects, whereas other anatomic sites were not universally imaged; hands (41 of 63 patients), ankles with or without feet (50 patients), lateral spine (44 patients), and lateral knee radiographs (33 patients) of patellae were available for some patients.
RESULTS
Mutation Analysis
Of the 63 probands, 55 (87%) were found to harbor either previously reported mutations (10 mutations in 35 patients; Table I) or a mutation considered to be pathogenic for the MED phenotype (18 mutations in 20 patients; Table II). Of these, 30 patients (55%) had a heterozygous MATN3 mutation, 23 (41%) a heterozygous COMP mutation, one (2%) a heterozygous COL9A2 mutation, and one (2%) compound heterozygous DTDST mutations. The most common mutation identified in this study was MATN3: c.361C > T (p.R121W), which was found in 20 patients (Table III).
| Gene | Exon | Nucleotide | Protein | Effect | Domain | No of cases | References |
|---|---|---|---|---|---|---|---|
| MATN3 | 2 | c.359C > T | p.T120M | Missense | VWFA, β-2 | 4 | a |
| MATN3 | 2 | c.361C > T | p.R121W | Missense | VWFA, β-2 | 20 | a b |
| COMP | 10 | c.1021_1026delGAGGAC | p.E341_D342del | Deletion | TSP type-3 3 repeat | 1 | c |
| COMP | 10 | c.1120_1122delGAC | p.D374del | Deletion | TSP type-3 4 repeat | 1 | d |
| COMP | 11 | c.1153G > A | p.D385N | Missense | TSP type-3 4 repeat | 4 | e |
| COMP | 13 | c.1318G > A | p.G440R | Missense | TSP type-3 6 repeat | 1 | f |
| COMP | 13 | c.1371_1373delGGA | p.E457del | Deletion | TSP type-3 7 repeat | 1 | g |
| COMP | 18 | c.2152C > T | p.R718W | Missense | TSP C-terminal domain | 2 | c e |
| DTDST | 2 | c.485_486delTG | p.V162GfsX12 | Nonsense | Transmembrane domain | 1i | h |
| DTDST | 3 | c.1153G > A | p.D385N | Missense | Transmembrane domain |
| Gene | Exon | Nucleotide | Protein | Effect | Domain | Known mutations at the same codon | Co-segregation in family | Sequence conserved among speciesi | In-silico analysis | Frequency in control (%) | No of cases | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphen | SIFT | Pmut | |||||||||||
| MATN3 | 2 | c.437T > G | p.L146R | Missense | VWFA, α-3 | None | 4/6 | PD | Affect | Neutral | 0 | 1 | |
| MATN3 | 2 | c.477C > T | p.G159G | Splicing | VWFA | None | 6/6 | NA | NA | NA | 0 | 1 | |
| MATN3 | 2 | c.575T > C | p.I192T | Missense | VWFA, β-4 | p.I192Na | Confirmed | 6/6 | PD | Affect | Neutral | 0 | 1 |
| MATN3 | 2 | c.626G > C | p.R209P | Missense | VWFA, α-5 | None | 4/6 | PD | Td | Neutral | 0 | 1 | |
| MATN3 | 2 | c.659T > C | p.V220A | Missense | VWFA, β-5 | None | Confirmedj | 6/6 | PD | Td | Neutral | 0.6 | 2 |
| COMP | 9 | c.874T > G | p.C292G | Missense | TSP type-3 1 | p.C292Wb | Confirmed | 5/5 | PD | Affect | Neutral | 0 | 1 |
| COMP | 9 | c.904G > T | p.D302Y | Missense | TSP type-3 2 | p.D302Vb | 5/5 | PD | Affect | Path | 0 | 1 | |
| COMP | 9 | c.949G > A | p.D317N | Missense | TSP type-3 2 | None | Mosaic motherk | 5/5 | PD | Affect | Neutral | 0 | 1 |
| COMP | 9 | c.955G > C | p.D319H | Missense | TSP type-3 2 | None | 5/5 | PD | Affect | Neutral | 0 | 1 | |
| COMP | 10 | c.1112G > A | p.C371Y | Missense | TSP type-3 4 | p.C371Sc | Confirmed | 5/5 | PD | Affect | Path | 0 | 1 |
| p.C371Fd | |||||||||||||
| COMP | 10 | c.1117_1122delGACGAC | p.D373_D374 del | Deletion | TSP type-3 4 | p.D372dele | 5/5 | NA | NA | NA | 0 | 1 | |
| COMP | 10 | c.1126G > C | p.D376H | Missense | TSP type-3 4 | p.D376Vf | Confirmed | 5/5 | Benign | Affect | Path | 0 | 1 |
| p.D376Ng | |||||||||||||
| COMP | 10 | c.1229G > A | p.C410Y | Missense | TSP type-3 5 | None | Confirmed | 5/5 | PD | Affect | Path | 0 | 1 |
| COMP | 11 | c.1252C > G | p.Q418E | Missense | TSP type-3 5 | None | 5/5 | PD | Affect | Neutral | 0 | 1 | |
| COMP | 13 | c.1372G > T | p.D458Y | Missense | TSP type-3 7 | None | 5/5 | PD | Affect | Path | 0 | 1 | |
| COMP | 13 | c.1444G > A | p.D482N | Missense | TSP type-3 7 | p.D482Gc | 5/5 | PD | Affect | Path | 0 | 2 | |
| p.D482Hh | |||||||||||||
| COMP | 14 | c.1519G > A | p.D507N | Missense | TSP type-3 8 | p.D507Gb | Confirmed | 5/5 | PD | Affect | Path | 0 | 1 |
| COL9A2 | IVS3 | c.186 + 1G > T | Splicing | Confirmed | 5/5 | NA | NA | NA | 0 | 1 | |||
- PD, probably damaging; Td, tolerated; Path, pathological; NA, not available.
- a Jackson et al. [2004].
- b Deere et al. [1999].
- c Susic et al. [1997].
- d Mabuchi et al. [2003].
- e Briggs et al. [1995].
- f Kennedy et al. [2005].
- g Zankl et al. [2007].
- h Song et al. [2003].
- i Refer to supplementary eFigure 1—see Supporting Information online.
- j Co-segregation with phenotype was confirmed in one family.
- k Asymptomatic mother seems to be a mosaic for this mutation.
| Patient no | Gender | Gene | Nucleotide | Agea | Height | Presenting symptomb | |
|---|---|---|---|---|---|---|---|
| cm | percentile | ||||||
| 1 | M | MATN3 | c.359C > T | 9 | 124 | 10 | Gait abnormality |
| 2 | M | MATN3 | c.359C > T | 10 | 123 | 1 | Hip pain |
| 3 | M | MATN3 | c.359C > T | 11 | 151 | 89 | Short stature |
| 4 | M | MATN3 | c.359C > T | 13 | 157 | 69 | Knee pain |
| 5 | F | MATN3 | c.361C > T | 3 | 90 | 35 | Affected father |
| 6 | M | MATN3 | c.361C > T | 5 | 102 | 21 | Leg pain |
| 7 | M | MATN3 | c.361C > T | 6 | 116 | 53 | Hip pain |
| 8 | M | MATN3 | c.361C > T | 8 | 121 | 32 | Knee pain |
| 9 | M | MATN3 | c.361C > T | 9 | 123 | 4 | Leg pain |
| 10 | M | MATN3 | c.361C > T | 10 | 131 | 22 | Gait abnormality |
| 11 | F | MATN3 | c.361C > T | 10 | 136 | 48 | Knee pain |
| 12 | M | MATN3 | c.361C > T | 11 | 137 | 16 | Hip pain |
| 13 | M | MATN3 | c.361C > T | 12 | 143 | 23 | Hip pain |
| 14 | F | MATN3 | c.361C > T | 13 | 150 | 20 | Genu varum |
| 15 | M | MATN3 | c.361C > T | 14 | 151 | 4 | Genu valgum |
| 16 | M | MATN3 | c.361C > T | 14 | 164 | 51 | Genu varum |
| 17 | M | MATN3 | c.361C > T | 15 | 160 | 13 | Genu valgum |
| 18 | M | MATN3 | c.361C > T | 15 | 164 | 31 | Genu valgum |
| 19 | M | MATN3 | c.361C > T | 15 | 173 | 85 | Knee pain |
| 20 | F | MATN3 | c.361C > T | 15 | 160 | 56 | Genu valgum |
| 21 | M | MATN3 | c.361C > T | 17 | 161 | 2 | Windswept deformity, knee |
| 22 | F | MATN3 | c.361C > T | 19 | 160 | 41 | Gait abnormality |
| 23 | F | MATN3 | c.361C > T | 21 | 163 | 67 | Hip pain |
| 24 | M | MATN3 | c.361C > T | 22 | 172 | 41 | Gait abnormality |
| 25 | M | MATN3 | c.437T > G | 12 | 142 | 19 | Genu valgum |
| 26 | M | MATN3 | c.477C > T | 11 | 142 | 56 | Hip pain |
| 27 | M | MATN3 | c.575T > C | 8 | 124 | 47 | Gait abnormality |
| 28 | F | MATN3 | c.626G > C | 7 | 120 | 51 | Genu valgum |
| 29 | M | MATN3 | c.659T > C | 9 | 140 | 84 | Knee pain |
| 30 | M | MATN3 | c.659T > C | 14 | 167 | 86 | Gait abnormality |
| 31 | F | COMP | c.874T > G | 17 | 140 | <0.5 | Knee pain |
| 32 | F | COMP | c.904G > T | 12 | 125 | <0.5 | Gait abnormality |
| 33 | M | COMP | c.949G > A | 12 | 145 | 48 | Gait abnormality |
| 34 | F | COMP | c.955G > C | 5 | 98 | 8 | Gait abnormality |
| 35 | M | COMP | c.1021_1026delGAGGAC | 10 | 115 | <0.5 | Knee pain |
| 36 | F | COMP | c.1112G > A | 15 | 144 | <0.5 | Hip pain |
| 37 | F | COMP | c.1117_1122delGACGAC | 5 | NA | NA | Foot deformity |
| 38 | F | COMP | c.1120_1122delGAC | 15 | 142 | <0.5 | Genu varum |
| 39 | M | COMP | c.1126G > C | 19 | 156 | <0.5 | Gait abnormality |
| 40 | F | COMP | c.1153G > A | 7 | 115 | 22 | Short stature |
| 41 | M | COMP | c.1153G > A | 13 | 150 | 24 | Gait abnormality |
| 42 | M | COMP | c.1153G > A, | 16 | 160 | 2 | Ankle pain |
| 43 | F | COMP | c.1153G > A | 21 | 153 | 7 | Hip pain |
| 44 | F | COMP | c.1229G > A | 9 | 122 | 18 | Waddling gait |
| 45 | M | COMP | c.1252C > G | 28 | 173 | 48 | Hip pain |
| 46 | F | COMP | c.1318G > A | 5 | 98 | <0.5 | Gait abnormality |
| 47 | M | COMP | c.1371_1373delGGA | 12 | 124.40 | <0.5 | Windswept deformity, knee |
| 48 | F | COMP | c.1372G > T | 6 | 109 | 23 | Hip pain |
| 49 | F | COMP | c.1444G > A | 12 | 141 | 3 | Gait abnormality |
| 50 | F | COMP | c.1444G > A | 29 | 148 | 1 | Gait abnormality |
| 51 | M | COMP | c.1519G > A | 15 | 160 | 10 | Genu valgum |
| 52 | M | COMP | c.2152C > T | 15 | 165 | 26 | Gait abnormality |
| 53 | F | COMP | c.2152C > T | 17 | 154 | 12 | Hip pain |
| 54 | F | CoL9A2 | c.186 + 1G > T | 12 | 143 | 13 | Knee pain |
| 55 | F | SLC26A2 | c.485_486 delTG, c.1153G > Ac | 10 | 131 | 16 | Genu valgum |
- NA, not available.
- a Age at the time of height measurement.
- b Main symptom among various symptoms.
- c Compound heterozygous.
All five novel MATN3 sequence variants identified in this study were located in the vWFA domain, and all 12 novel COMP variants in the TSP type-3 domain. Seven novel sequence variants were predicted to substitute amino acid at the same or adjacent codons affected by previously reported mutations. Co-segregation with the phenotype in a family was confirmed for eight variants. All 12 novel sequence variants of the COMP gene and four of six of the MANT3 gene were located at amino acids well conserved among species (Supplementary eFigure—see Supporting Information online). Fourteen of the 17 novel sequence variants with possible amino acid substitution were predicted to be pathogenic by at least two of PolyPhen, SIFT, or PMut.
Seventeen of 18 novel sequence variants were not detected in 90 normal controls (180 alleles), but MATN3 c.659T > C (p.V220A) was found in two of 168 normal controls. Co-segregation of this sequence variant with a MED phenotype was confirmed in one family. MATN3 c.477C > T (p.G159G) was considered to be pathogenic despite being a synonymous change because this nucleotide substitution was predicted to generate a novel presumptive splicing acceptor site at nucleotide positions 476–477 by GeneSplicer, MaxEntScan, and SpliceSiteFinder. However, we were not able to corroborate this by mRNA study because no MATN3 expressing tissue could be obtained from the patient concerned.
Radiological Findings
Patients harboring mutations of MATN3 (30 patients) and COMP (23 patients) accounted for the majority of patients with a molecularly confirmed diagnosis and thus, the radiologic findings of those two groups were compared to delineate radiologic phenotypes. Radiographic findings are summarized in Table IV.
| Gene | P-valuea | ||
|---|---|---|---|
| COMP | MATN3 | ||
| No. of patients | 23 | 30 | |
| Age (year) mean ± SD | 11.0 ± 6.4 | 9.7 ± 2.7 | 0.3810 |
| Range | 4–28 | 2–13 | |
| Radiological findings | Patient number (%) | ||
| Hip | |||
| Irregular, shallow, and straight acetabular roof | 19 (83) | 9 (29) | 0.0001 |
| Acetabular marginal spur | 15 (65) | 0 (0) | <0.0001 |
| Flat and crescent femoral head | 13 (57) | 29 (97) | 0.0004 |
| Small and round femoral head | 7 (30) | 0 (0) | 0.0012 |
| Irregular, dysplastic femoral head | 3 (13) | 1 (3) | 0.1847 |
| Absent femoral head | 0 (0) | 1 (3) | 0.3767 |
| Wide femoral neck | 22 (96) | 26 (87) | 0.2674 |
| Hip joint narrowing | 9 (39) | 7 (23) | 0.2144 |
| Irregular contour of iliac crest and ischium | 6 (26) | 0 (0) | 0.0030 |
| Coxa valga | 15 (65) | 24 (80) | 0.2264 |
| Coxa vara | 7 (30) | 6 (19) | 0.3815 |
| Knee | |||
| Metaphyseal flaring of the distal femur and proximal tibia | 18 (78) | 25 (83) | 0.6399 |
| Metaphyseal vertical striations | 3 (13) | 27 (90) | <0.0001 |
| Fragmented or cracked epiphyses of the femur and tibia | 8 (35) | 0 (0) | 0.0005 |
| Shallow intercondylar notch | 8 (35) | 8 (27) | 0.5236 |
| Medial beaking of the tibial metaphysis | 14 (61) | 12 (39) | 0.1320 |
| Genu valgum | 11 (48) | 17 (55) | 0.5228 |
| Genu varum | 11 (48) | 12 (39) | 0.5688 |
| Joint narrowing | 15 (65) | 25 (81) | 0.1287 |
| Small, thin patella | 11/16 (69)b | 11/18 (61) | 0.6418 |
| Double patella | 1/16 (6) | 0/18 (0) | 0.2817 |
| Hand | |||
| Delayed carpal bone age | 12 (52) | 5/21 (24) | 0.0536 |
| Ragged, irregular contour of carpal bones | 19 (83) | 0/21 (0) | <0.0001 |
| Bachydactyly | 13 (57) | 2/21 (10) | 0.0010 |
| Irregular or flat distal radiul/ulna epiphyses | 14 (61) | 10/21 (48) | 0.3779 |
| Metaphyseal flarring/irregularity | 11 (48) | 3/21 (14) | 0.0170 |
| Ankle | |||
| Irregular, flat tibial epiphysis | 20/22 (91) | 25/29 (86) | 0.6057 |
| Ragged, irregular contour of tarsal bones | 15/22 (69) | 2/29 (7) | <0.0001 |
| Tibial metaphysis vertical striations | 5/22 (23) | 24/29 (83) | <0.0001 |
| Dital tibia/fibula metaphyseal irregularity/striations | 15/22 (68) | 10/29 (34) | 0.0171 |
| Spine | |||
| Mild platyspondyly | 2/22 (9) | 9/23 (39) | 0.0191 |
| Vertebral end plate irregularity | 12/22 (54) | 8/23 (35) | 0.1823 |
| Convex end plate with/without central beaks of vertebral bodies | 8/22 (36) | 1/23 (4) | 0.0073 |
- MED, multiple epiphyseal dyaplasia; COMP, cartilage oligomeric matrix protein; MATN3, Matrilin-3.
- a Age was compared using Student's t-test, and the others by Fisher's exact test.
- b When a specific radiograph was not available in all patients, the result was described as fractional number. The denominator means number of patients whose radiograph was available.
COMP Mutation
Regardless of age at radiographic examination, all patients showed abnormally shaped femoral heads. Patterns of abnormally shaped capital femoral epiphyses were small and round in seven patients (30%; Fig. 1A–C), dysplastic and fragmented in three (13%; Fig. 1D), and flat and crescent shaped in 13 (57%; Fig. 1E). A round shape was seen in patients between the ages of 4 and 9 years (inclusive), whereas a flat, crescent, and/or dysplastic shape was observed in patients aged 10–28 years. The femoral neck was broad in most patients and tended to be in valgus deformity. In three adult patients, the femoral neck appeared to be short and in varus deformity (Fig. 1F). The acetabular contour was irregular and showed loss of normal concavity with a steep acetabular angle and marginal spurs (Fig. 1A,B,D). One interesting finding was of an irregular contour of the iliac crest and ischium, which was noted in six patients (26%) between the ages of 6–12 years (Fig. 1D).
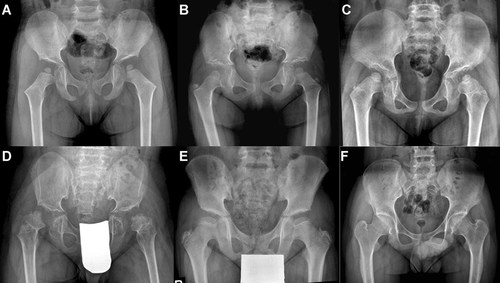
Radiographic features of hips in patients with COMP mutations. A: Patient 37 at 5 years showing small and round femoral heads. The acetabular roofs are shallow and steep with marginal spurs. The femoral necks are broad and showing coxa valga. B: Patient 48 at 6 years showing round femoral heads. The acetabular roofs are flat and irregular. C: Patient 32 at 9 years. The capital femoral epiphyses are too small for age and rounded. The acetabular roofs are shallow and showing irregular contours. The femoral necks are broad. D: Patient 47 at 11 years showing the most significantly affected hips with small and dysplastic appearing capital femoral epiphyses. The acetabular roofs are flat and irregular with marginal spurs. Contour irregularities of the ilium and ischium are shown. The ossification centers of the great trochanters are small for age. E: Patient 51 at 12 years showing small and crescent shape of femoral heads. The acetabular roofs are flat and shallow but contour irregularity is minimal. The greater trochanters shows dysplastic shape. F: Patient 39 at 19 years. The femoral heads showing thin crescent shape. The acetabular roofs are essentially normal but joint space narrowing is seen. The femoral necks are short.
All patients had flat and irregularly shaped epiphyses of the distal femur and proximal tibia (Fig. 2). Because distal femoral epiphyses were flat, femoral intercondylar notches became shallow, which was the major finding in subjects aged between 11 and 28 years (Fig. 2B,D). Fragmented and cracked epiphyses were observed in the distal femur in eight patients (35%) and proximal tibia in four (17%). At the knees, thin, irregular contours of the medial and lateral borders of the proximal tibial epiphysis and a medial beak at the metaphysis were the most consistent findings (61%; Fig. 2F). Lateral knee radiographs were available in 16 patients, and of these, 11 (69%) had small and thin patella. One patient had a double-layered patella (Fig. 2G).
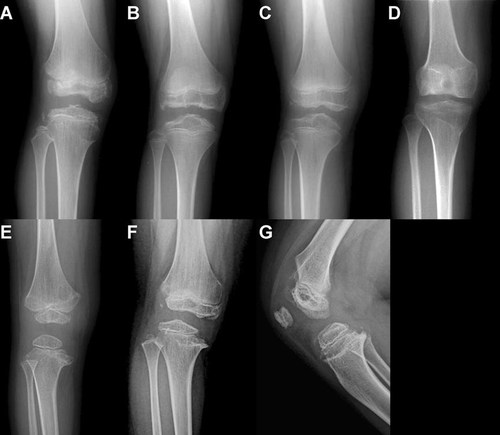
Radiographic features of knees in patients with COMP mutations. A: Patient 31 at 11 years. The epiphyses at the knees are small and irregular. Fragmentation and/or cleavage of ossification centers are shown. There are vertical striations at distal femoral and proximal tibial metaphyses. B: Patient 42 at 12 years. The epiphysis of the distal femur is flatten and irregularities of the articular surface of the femoral condyles are seen. The proximal tibial epiphysis shows irregular contour and flattening of medial and lateral sides. C,D: Patient 36 at 11 and 15 years. There is flattening of the distal femoral epiphysis, more severely affected at the lateral aspect. A small fragmented ossicle is shown. After physeal closure at age 15 years, irregularity of the articular surface disappeared. Flatness of the femoral condyles with shallow intercondylar notch is shown. Depression and flatness of tibial plateau is shown. E: Patient 46 at 5 years showing metaphyseal flaring of the distal femur with irregular contour of the epiphysis. Metaphyseal vertical striations are also observed. The proximal tibial metaphysis showing irregular articular surface and medial beaking. The tibial epiphysis is small but showing smooth contour. F,G: Patient 32 at 9 years showing typical epiphyseal changes of the distal femur and the proximal tibia. Metaphyseal beaking of the proximal tibia is evident. Lateral knee radiograph of the same patient showing double-layered patella.
Hand radiographs were available for 23 patients. Carpal bones were small and had ragged contours in 19 (83%), 14 (61%) had an irregular and flat distal radial/ulnar epiphyses, 13 (57%) had brachydactyly, and 12 (52%) had a delayed carpal bone age (Fig. 3). Ankle radiographs, including feet or not, were available for 22 patients. The most common finding was a thin and irregularly shaped distal tibial epiphysis in 20 (91%). A ragged contour of tarsal bones was also observed in 15 patients (68%; Fig. 3B).
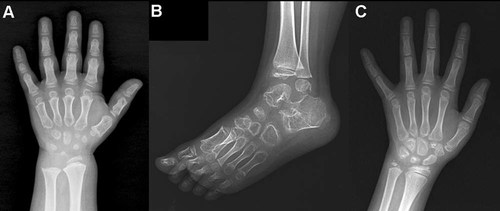
Hand and foot radiographs of the patients with COMP mutations. A,B: Patient 48 at 6 years. Hand shows brachymetacarpia with distal metaphyseal irregularities and roundness of the epiphyses. The carpal bones are small and irregular, and carpal bone age is delayed. Epiphysis of the distal radius is small and irregular. Mild metaphyseal flaring is seen at distal radius and ulna. Oblique foot radiograph shows irregular epiphyses of the distal tibia and fibula with fragmentation. Contours of the calcaneus, talus, and tarsal bones are irregular. C: Patient 51 at age 12 years. There is no brachydactyly. Carpal bones are small and showing contour irregularities, and delayed bone age. Epiphyses of the distal radius and ulna are irregular and dysplastic.
Twenty-two of the 23 patients had lateral spine radiographs. Vertebral body heights were usually normal though mild flattening was present in two patients. However, the end plates of vertebral bodies were irregular in six (27%) patients. Patients aged between 4 and 6 years showed convex end plates and a rounded vertebral body appearance.
MATN3 Mutation
The capital femoral epiphyses were flat and crescent-shaped in 23 patients (77%; Fig. 4A,D,E) with a MATN3 mutation, asymmetrically or asymmetrically flattened with a fragmented appearance in six (20%; Fig. 4B,C), and non-ossified in one (3%; Fig. 4F). In contrast to the COMP mutation, no patient aged <9 years had a small, round femoral head. Most of the patients had preserved normal concavity of the acetabular roof. An irregular acetabular contour was present in nine (29%; Fig. 4E).
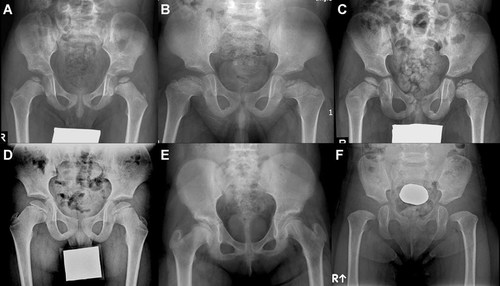
Radiographic features of hips in patients with MATN3 mutations. A: Patient 6 at 5 years showing small and thin femoral heads, and normal acetabular roofs. The femoral necks are broad. B: Patient 7 at 6 years showing crescent shape femoral heads with fragmentation bilaterally. The acetabular roofs appear to be normal. The femoral neck is short and broad. C: Patient 27 at 6 years. Dense, crescent shape of the femoral heads with fragmentation on the left side mimics Legg-Calve-Perthes disease. D: Patient 29 at 9 years showing crescent shape femoral heads with mild flattening of its medial aspect. The acetabular roofs are normal. The femoral necks are broad. E: Patient 14 at 12 years. The femoral heads showing typical thin and crescent shape. The acetabular roof are showing irregular contour. Sclerotic vertical striations are seen in the left femoral neck. F: Patient 5 at 2 years. No ossification of femoral heads is shown. The femoral necks are broad. The acetabular roofs appear to be normal.
The epiphyses of the distal femur and proximal tibia were flat and irregular, but thinning of the epiphyses in the distal femur was more severe at the lateral side of the epiphyses, whereas, the proximal tibial epiphyses thinned out at both sides, to produce a triangular shape or a shape resembling a harlequin's hat (Fig. 5A,B,E,G). However, unlike COMP mutations, no patient showed a fragmented or cracked epiphyses. Vertical metaphyseal striations in the distal femur and proximal tibia were observed in 26 of 30 patients (87%). One of these patients underwent MRI of the knee and also showed longitudinal striations (Fig. 5E,F). Lateral knee radiographs were available for 18 out of 30 patients and the patellae were found to be thin and to have irregular contours.
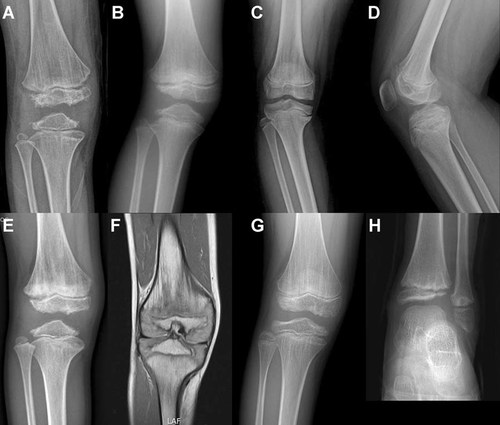
Radiographic features of knees in patients with MATN3 mutations. A: Patient 2 at age 8 years. There are irregular and thin epiphysis of the distal femur and proximal tibia, especially the medial and lateral sides. Metaphyseal vertical striations are distinctly shown in the distal femur and some in proximal tibia. B: Patient 17 at 10 years showing small and irregular epiphysis of the distal femur, especially more thinning and hypoplastic appearance of the lateral aspect. The epiphysis of the proximal tibia shows thinness at both sides, resembling a triangular shape. Metaphyseal vertical striations are shown in the distal femur. C,D: Patient 14 at 12 years. Fomoral condyles are flat and shallowness of the intercondylar notch is seen. Medial flattening of the proximal tibial epiphysis is shown. Vertical striations of the distal femur and proximal tibia are seen. Lateral knee radiograph of the same patient also showing vertical striations in distal femur. E,F: Patient 9 at 9 years showing irregular epiphyses of the distal femur and proximal tibia, with thinning of the epiphyses at both sides. Dense and thick metaphyseal striations are shown in the distal femur. T1-Weighted coronal MR imaging also shows vertical striations as hyposignal intensity in the distal femur. G,H: Patient 12 at 11 years showing distinct vertical striations in the distal femur and proximal tibia. Metaphyseal irregularityies with short striations are also shown in the distal tibia of the same patient. The distal tibial epiphysis is flat and irregular, especially the lateral aspect.
Hand radiographs were available for 21 patients. Delayed carpal bone age was observed in five (24%). In contrast to the COMP mutation for which carpal bones had a ragged contour, the carpal bones of the MATN3 patients were smooth. Brachydactyly was observed in two patients. The epiphyses of distal tibiae were flat and irregular in shape and concordant metaphyseal irregularity was noted (Fig. 5H). Lateral spine radiographs in 23 patients showed mild platyspondyly and end-plate irregularities in approximately one third (Fig. 6).
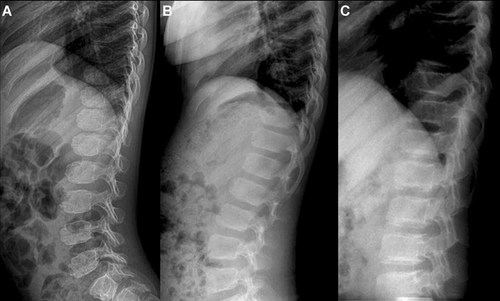
Radiographic features of the spine in patients with COMP or MATN3 mutations. A: Patient 46 with COMP mutation at 5 years. Bulging of the superior and inferior endplates brings roundness of the vertebral bodies. B: Patient 33 with COMP mutation at 9 years showing mild flattening of the vertebral bodies. C: Patient 15 with MATN3 mutation at 12 years shows irregularities of the vertebral endplates.
DISCUSSION
MED is genetically heterogeneous and this makes molecular diagnosis difficult. Overall detection rates for MED mutations in European surveys range from 25% to 50% [Briggs and Chapman, 2002; Jakkula et al., 2005; Kennedy et al., 2005]. These low detection rates may be due in part to misdiagnosis, and in this context, preselection of MED cases by expert clinical and radiological review could enhance the mutation detection rate to 81% [Zankl et al., 2007]. In the present study, preselection of MED patients for mutation analysis by a radiological review panel resulted in a detection rate of 87%. Accordingly, the results of these two studies indicate that careful subject preselection can increase mutation detection rates appreciably.
The reported frequencies of the six known genes in MED patients vary considerably, probably because of selection bias. The contribution of the COMP locus to MED is 30–50%, followed by DTDST at about 25%, and the remainder is split between MATN3 and COL9A1,2 and 3 in European populations [Jakkula et al., 2005; Unger et al., 2008], but another study reported that the DTDST mutation was most common in molecularly defined MED [Ballhausen et al., 2003]. However, frequencies found in survey conducted in East Asia differ from those found in Western surveys. Comprehensive screening for MED mutations in the Japanese population showed that mutations in MATN3 were the most frequent (23%), followed by COMP (20%), and type 9 Collagen genes (9%), whereas no DTDST mutations were found. Our study demonstrates that the mutation spectrum of MED in Koreans is similar that reported in the Japanese survey, that is, the MATN3 mutations were most prevalent, and was present in 45% of our cohort, and the frequency of the COMP mutation was 35%, which is similar to that reported in Europeans. However, the frequency of mutations in COL9A1-3 and DTDST were very low and identified only in one patient each. These findings regarding the nature of the mutation spectrum in Korean MED patients suggest that a different molecular screening strategy should be applied in the population-based survey.
This study reveals MATN3: c.361C > T is the most common mutation in Korean MED patients, and that it accounts for approximately 67% (20/30) of patients harboring MATN3 mutations. The c.361C > T (p.R121W) mutation was the most frequently detected MATN3 mutation in a Japanese study as well, but it was found in 38% (three of eight patients) [Mabuchi et al., 2004]. The high prevalence of this specific mutation may be the main reason for the observed ethnic differences between the mutation spectra of East Asians and Europeans.
Degrees of severity of radiographic manifestations of affected joints within same mutation groups were not identical because ages at radiographic examinations differed. We observed three patterns of a misshaped femoral head for COMP mutations, that is, a small and round shape, a flat and crescent-like shape, and a dysplastic and/or fragmented shape. Of these, a round shape of the capital femoral epiphysis, referred to as a “mini-epiphysis,” has been reported to be a characteristic finding of COMP mutations [Unger et al., 2001, 2008; Lachman et al., 2005]. This finding was observed in 39% of patients with COMP mutations, but was confined to patients aged 4–9 years. A small and rounded femoral head seems to become crescent-shaped or dysplastic and/or fragmented appearance with age, but this suggestion is limited by the fact that the radiographs of patients with different ages were compared, and not sequential radiographs of same patients. A larger study with follow-up radiographs in same patients is required to explore this suggestion. On the other hand, patients with MATN3 mutations, even those aged <9 years, did not have a small and round femoral head. Furthermore, the majority of patients (97%) with MATN3 mutations had either symmetrical or asymmetrical crescent shaped femoral head. This observation suggests that a small and round femoral head is observed in patients suspected to have MED, the COMP mutation should be the first gene examined.
When a femoral head has a symmetrical or asymmetrical crescent shape with fragmentation, radiographic abnormalities may overlap with bilateral Legg-Calve-Perthes disease (LCPD). In fact, some of our patients were initially suspected to have bilateral LCPD or Meyer disease (Fig. 4B,C). However, careful clinical examination and radiographic evaluations of knee and ankle joints can exclude bilateral LCPD. In our skeletal survey of 55 patients, non-ossified capital femoral epiphysis was observed in only one patient who had a MATN3 mutation (Fig. 4F); however, the radiographic examination was performed at 2 years of age, and absence of ossification may be due to delayed in appearance for ages.
Acetabular changes were a distinctive feature in the hip joints of patients with COMP mutations. In a previous study, an irregular acetabular contour was described as radiographic change associated with the presence of COMP mutation [Lachman et al., 2005]. We analyzed acetabular contours in more detail. Straight and shallow acetabular roofs, indicating loss of normal concavity, were observed in the 19 out of 23 patients (83%) with COMP mutations. In addition, marginal spikes of the acetabulum were noted in 65%, which may be the consequence of acetabular irregularities. Acetabular contour irregularities and shallowness were not observed in three adult patients, and thus acetabular changes seem to be age-dependent alteration. In contrast to the COMP mutations, acetabular irregularities were less frequent (29%) in patients with MATN3 mutations. Acetabular contours also should be carefully evaluated in the hip radiographs of MED patients, as this could provide additional indications of genotype.
Knee joints consistently showed dysplastic epiphyses and metaphyses of the distal femur and proximal tibia, regardless of genotype. A fragmented or cracked distal femoral epiphysis was observed in 35% of patients with COMP mutations, and this was not observed in any patient with MATN3 mutations. Unger et al. [2008] described a cracked distal femoral epiphysis as the “glacier crevice” sign, and found this helpful for diagnosing the presence of COMP mutation. In the present study, a cracked epiphysis was primarily seen in the distal femur, but the proximal tibial epiphysis was also affected in four patients. A flat distal femoral epiphysis tended to result in a flat intercondylar eminence and shallow intercondylar notch with age. This finding was exclusively observed in three adult patients and frequently seen in COMP mutations around the age of physeal closure, but also observed in patients with MATN3 mutations.
Makitie et al. [2004] described vertical striations in their report on MATN3 mutations in 12 patients. In the present study, this finding was observed in 26 of 30 patients (87%) with MATN3 mutation, and in 13% (three of 23) patients with COMP mutation. Our study shows that when vertical striations are present in the distal femoral and/or proximal tibial metaphyses with distinct epiphyseal changes, the probability of MATN3 mutation increases.
Previous studies have reported that a double-layered patella is found exclusively in patients with the DTDST mutation, and thus, suggested that this feature be considered pathognomonic [Makitie et al., 2003; Superti-Furga et al., 1999]. However, other studies have reported a double-layered patella in a patient with COL9A2 mutation and in a pseudoachondroplasia patient with COMP mutation [Nakashima et al., 2005; Vatanavicharn et al., 2008]. In the present study, one patient with COMP mutation had a double-layered patella. Thus, when a double-layered patella is found, diagnostic sensitivity for the DTDST mutation should be considered high, but not exclusive.
Radiographic evaluations of the hands in MED patients should focus on bone age and the contours of carpal bones. A delayed carpal bone age was evident in around half of the patients with COMP mutation and in 23% of patients with MATN3 mutation. Ragged and irregular contours of carpal bones are predominated (83%) in patients with COMP mutations, whereas this finding was never observed in MATN3 mutations. The presence of brachydactyly and small, round metacarpal epiphyses are frequently associated with COMP mutations [Unger et al., 2001; Lachman et al., 2005]. Over half of the patients (57%) with COMP mutation in our series had brachydactyly, whereas two patients (9%) with MATN3 mutation had radiographic evidence of brachydactyly.
The term “multiple epiphyseal dysplasia” was coined at a time when spinal involvement was not recognized. However, experience shows that some patients have accompanying mild spinal dysplasia, such as, endplate irregularities and/or a slight decrease in vertebral body height [Mabuchi et al., 2004]. In the present study, endplate irregularities with normal vertebral body heights or mild platyspondyly were observed in 55% of patients with the COMP mutation and in 35% with the MATN3 mutation. However, oval-shaped vertebral bodies (superior and inferior endplate rounding) were also observed in patients aged between 4 and 7 years with the COMP mutation, and thus, it is evident that some spinal alteration may accompany in patients with COMP and MATN3 mutations.
With next generation sequencing techniques, multi-gene sequencing will become less cumbersome but molecular interpretation will become more complicated. Thus, detailed evaluation of radiological findings will enhance suspicion of the presence of particular gene mutations in MED patients and facilitate the planning of genetic studies.




