Clinical and molecular description of a Wilms tumor in a patient with tuberous sclerosis complex†
How to Cite this Article: Spreafico F, Notarangelo LD, Schumacher RF, Savoldi G, Gamba B, Terenziani M, Collini P, Fasoli S, Giordano L, Luisa B, Porta F, Massimino M, Radice P, Perotti D. 2011. Clinical and molecular description of a Wilms tumor in a patient with tuberous sclerosis complex. Am J Med Genet Part A 155:1419–1424.
Abstract
We report on a girl affected with tuberous sclerosis, carrying a germline de novo TSC2 mutation, c.4934-4935delTT, leading to a p.F1645CfsX7, who developed a unilateral Wilms tumor (WT). Molecular investigation of the tumor biopsy at diagnosis revealed the loss of the constitutional wild-type TSC2 allele, and loss of heterozygosity for the WT1 gene. Deletion of the WTX gene was also present, but it involved the functionally inactive X chromosome. No mutation affecting the remaining WT1 and WTX alleles, as well as the CTNNB1 gene was found. Pathological examination of the surgical specimen documented the presence of diffuse anaplasia and p53 immunoreactivity. To the best of our knowledge, this is the second report of a patient with tuberous sclerosis who developed a WT, and it represents the first case in which a detailed clinical and molecular description is provided. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Tuberous sclerosis complex (TSC, OMIM# 191100 and # 613254) is a rare autosomal dominant tumor-suppressor-associated syndrome, with an incidence at birth of approximately 1:10,000 [Siroky et al., 2009]. It is a multisystem disorder characterized by widespread hamartomas in several organs, including the brain, heart, skin, eyes, kidney, lung, and liver [reviewed by Crino et al., 2006; Curatolo et al., 2008]. The genes involved in this condition are TSC1, on chromosome 9q34, which codes for the protein product hamartin [van Slegtenhorst et al., 1997], and TSC2, located at position 16p13.3, coding for the protein tuberin [European Chromosome 16 Tuberous Sclerosis Consortium, 1993]. The frequency of mutations reported in TSC2 is consistently higher than in TSC1. Considering both familial and sporadic cases, TSC2 mutations are found in about 60% and TSC1 mutations in about 19% of TSC patients [Northrup and Au, 2009].
Phenotypic expression of the disease occurs on a genetic background of a germline inactivating mutation in the TSC1 or TSC2 gene, when there is a second somatic mutation involving the same locus. Most “second hit” mutations are deletions: loss of heterozygosity (LOH) in TSC1 or TSC2 has been consistently observed in the majority of TSC-associated renal angiomyolipomas, cardiac rhabdomyomas, subependymal giant-cell tumors, and lymphangiomyomatosis cells, but has only been rarely found in cerebral cortical tubers [reviewed by Crino et al., 2006; Curatolo et al., 2008].
The renal phenotypes of TSC include angiomyolipomas, oncocytomas, and polycystic kidney disease in TSC patients affected with the contiguous gene deletion syndrome involving both TSC2 and PKD1. Rarely, renal cell carcinomas occur in children and adults. These manifestations are pathologically distinct, deriving from epithelial (cysts, oncocytomas, and carcinomas) or mesenchymal tissues (angiomyolipomas) [Siroky et al., 2009].
We report on the clinical and molecular description of a girl affected with TSC who developed a Wilms tumor (WT), the most frequent malignant renal tumor in children.
WT is characterized by a high degree of genetic heterogeneity. The main genes involved in its pathogenesis and found to be mutated are WT1, located on chromosome 11p13 [Bonetta et al., 1990; Call et al., 1990; Gessler et al., 1990], and WTX mapped to chromosome Xq11.1 [Rivera et al., 2007]. Other known anomalies in WT include activating mutations in the β-catenin gene (CTNNB1) on chromosome 3p22.1, which are closely associated with WT1 mutations [Maiti et al., 2000], and mutations in the tumor protein p53 gene (TP53), on chromosome 17p13.1, which are associated with anaplastic histology [Bardeesy et al., 1994].
To the best of our knowledge, our patient represents the second case in which the association of these two rare conditions is reported.
CLINICAL REPORT
The proposita is a 3 9/12-year-old girl, first child of unrelated healthy Caucasian parents. The mother was 42 years old at the birth of the patient, and had three healthy children from a previous marriage; the father was 48 years old, and this was his first child. There were no known genetic diseases in either family. During pregnancy, the mother was not exposed to ionizing radiation and was not aware of any other teratogenic risk. Pregnancy was complicated by threat of spontaneous abortion at 7 weeks gestation. Due to advanced maternal age, villocentesis was performed in the 13th week, and resulted in a normal female karyotype (46,XX). At 33 weeks gestation, fetal sonography revealed at least three rhabdomyomas, in the interventricular septum of an otherwise healthy heart, suggestive of TSC. No other abnormalities were documented before birth.
Spontaneous delivery occurred at 37 weeks gestation. Birth weight was 2,530 g (10th centile), length was 45 cm (3rd centile), and head circumference was 33 cm (3rd–10th centile). Neonatal examination showed normal skin without hypopigmented areas, and no other clinical signs of TSC. Echocardiography confirmed the rhabdomyomas, the larger one obstructing the left ventricular outflow by at least 60%.
Abdominal sonography revealed a hyperechogenic area in the medulla of the upper pole of the right kidney. A cerebral magnetic resonance imaging (MRI) performed at birth showed numerous subependymal nodules, consistent with the diagnosis of TSC. At age 2 months, ophthalmologic examination revealed an eccentric pupil with a pigmented spot in the left eye and the patient started to develop hypopigmented areas on her lower limbs and on her back.
At the same time, she presented with focal motor seizures and epileptic spasms partially responsive to antiepileptic drugs: vigabatrin (GVG) at the beginning, followed by carbamazepine. The neurological evaluation was normal.
At age 14 months, follow-up abdominal ultrasound revealed a solid mass (9 cm) in the lower right kidney. This lesion was interpreted as benign by the responsible physician, possibly related to TSC. Five months later, following the development of macrohematuria, the child was transferred to the University Children's Hospital in Brescia, where a computed tomography (CT) scan confirmed a mass involving the right kidney (maximum diameter 13 cm), and possible retroperitoneal lymphadenopathy. Lung CT revealed two metastases. Abdominal open surgical biopsy was performed and showed a WT without anaplasia. Blastemal and epithelial components of WT were represented and no morphological evidence of angiomyolipoma was found (Fig. 1, panels A,B). Immunohistochemistry for WT1, using both WT clone WT49 (Monosan, the Netherlands) (Fig. 1, panel C) and WT1 (C-19, Santa Cruz Biotechnology, Heidelberg, Germany) antibodies was positive. No reactivity for the angiomyolipoma-related transcription factor E3 (TFE3), using the TFE3 (P-16) antibody (Santa Cruz Biotechnology) was found (Fig. 1, panel D).
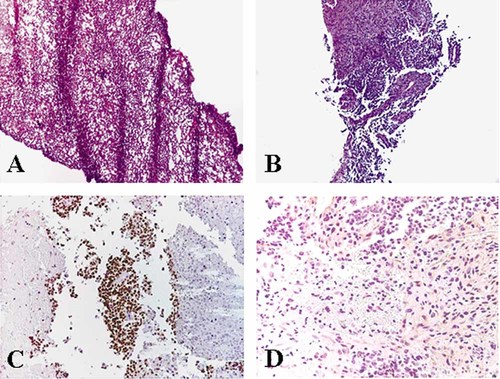
Biopsy material of the renal tumor. Panel A: Hematoxylin and eosin control section of the frozen material used for molecular analyses, representing blastemal component without anaplasia of nephroblastoma. Panel B: Hematoxylin and eosin control section of the formalin-fixed paraffin-embedded material, representing blastemal and epithelial components without anaplasia of nephroblastoma. Panel C: Presence of immunoreactivity for WT1. Panel D: Absence of immunoreactivity for TFE3.
The patient was treated with preoperative three-drug chemotherapy (vincristine, dactinomycin, doxorubicin). The underlying clinical problems prompted dose adjustments. After completing 4 weeks of chemotherapy demonstrating partial response (9 cm × 5.5 cm × 5 cm), radical nephrectomy was performed. Histologically, the tumor mass displayed post-treatment changes in 70% of the neoplasm. The residual viable tumor was represented by WT, and showed 85% epithelial, 10% stromal, and 5% of blastematous components. Diffuse anaplasia was present, in areas showing marked and diffuse p53 immunoreactivity in the nuclei of the neoplastic cells (DO-7 monoclonal p53 antibody; Novocastra, Newcastle upon Tyne, UK). No evidence of angiomyolipoma was found. Local stage III was assigned due to multiple lymph node metastases. Postoperative chemotherapy was intensified as per high-risk regimen (alternating the drug pairs carboplatin/etoposide and ifosfamide/doxorubicin), and radiotherapy was given. No major chemotherapy or radiotherapy-related unexpected side effects were reported.
MRI of the central nervous system showed numerous subependymal nodules and tuberous lesions in the cortex, without progression or signs of astrocytoma. At present, the patient presents asymmetric spasms associated with focal seizures occurring many times a month despite therapy with GVG and oxacarbamazepine. The standard neurological evaluation is still normal while her developmental profile shows a mild delay in achieving developmental milestones and the electroencephalographic pattern is compatible with the patient's cerebral malformations. Twenty-six months after the diagnosis of the WT, the patient is in complete remission. No relapse occurred at the original tumor site and the chest X-ray was negative for metastasis.
MATERIAL AND METHODS
DNA Analyses
The DNA from peripheral blood leukocytes (PBLs) of the patient and of her parents, and from the patient's WT biopsy was extracted as previously described [Perotti et al., 2004].
TSC1 and TSC2 Germline Mutation Screening
Investigation of all the exons of the TSC1 and TSC2 genes was performed on the patient's PBL DNA, and mutation screening was carried out with the automated WAVE 3500A Nucleic Acid Fragment Analysis System (Transgenomic, Inc., Omaha, NE). The WAVEMAKER 4.1 software was used to determine the optimal melting temperatures for the PCR amplified fragments (primers and condition available on request). Following PCR amplification, 10 µl of denaturated PCR products were injected into a high-throughput DNASep column and eluted with a linear acetonitrile gradient at a flow rate of 0.9 ml/min. PCR products with elution profiles of heterozygous fragments, showing multiple peaks and/or aberrant shaped peaks with respect to the homozygous fragments, were sequenced in both directions. Sequencing reactions were performed on amplification products using the ABI PRISM BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA) and examined on an ABI PRISM 3130 DNA Sequencer (Applied Biosystems), using the Sequencing Analysis software (Applied Biosystems).
TSC2 Gene Analysis in the WT
Allelic constitution at the TSC2 locus in the tumor was investigated comparing the electropherogram obtained from tumor and PBL DNAs after direct sequencing of the TSC2 exon in which the germline mutation was identified.
WT1 Gene Loss of Heterozygosity and Mutation Analyses
HinfI and HhaI restriction fragment length polymorphisms (RFLPs) at the WT1 locus were analyzed by PCR-mediated DNA amplification, followed by enzymatic digestion, as previously described [Perotti et al., 2005]. The GT dinucleotide repeat in the 3′ non-coding region of WT1 exon 10 was amplified by PCR using a 5′ end labeled primer, and amplified fragments were resolved on a 3130 Genetic Analyser (Applied Biosystems) and examined with the Genemapper Software 4.0 (Applied Biosystems) as already reported [Perotti et al., 2005, 2008].
The entire coding region of WT1 was PCR amplified from tumor DNA [Perotti et al., 2005] and PCR products were analyzed by sequencing as described above.
WTX Gene Loss of Heterozygosity and Mutation Analyses
The microsatellite sequences Fam123b-1 and Fam123b-2, located in intron 1 and approximately 350 bp downstream the termination codon of the WTX gene, respectively, were investigated as reported in detail [Perotti et al., 2008]. The localization of the WTX anomaly on the active or inactive X chromosome (Xa and Xi, respectively) was performed by a HUMARA assay as described [Perotti et al., 2008]. Briefly, the assay is based on DNA digestion with the methylation-sensitive restriction endonucleases HpaII and HhaI followed by PCR amplification of the region containing both the polymorphic CAG repeat in the first exon of the AR gene and flanking recognition sites for the above enzymes. Only methylated DNA, which is resistant to enzymatic digestion, gives rise to an amplification product.
The entire coding region of WTX was PCR amplified from tumor DNA [Perotti et al., 2008] and PCR products were analyzed by sequencing as previously described.
CTNNB1 and TP53 Genes Mutation Analyses
The exons 3, 7, and 8 of the CTNNB1 gene and exons 4–9 of the TP53 gene were amplified by PCR from tumor DNA and sequenced as described above (primers and condition available on request).
RESULTS
Molecular investigation of the TSC1 and TSC2 loci performed on the constitutional DNA of the patient revealed a germline c.4934-4935delTT leading to a p.F1645CfsX7 at the TSC2 locus (Fig. 2, panel A), thus confirming the clinical diagnosis of TSC.
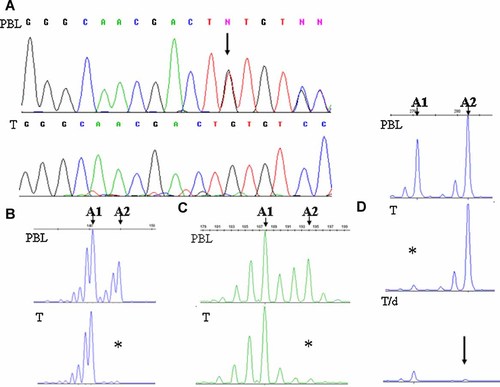
Molecular characterization of the TSC2, WT1, and WTX genes in the patient. Panel A: Germline TSC2 gene mutation present in the peripheral blood leukocytes (PBL) of the patient in heterozygosity, (arrow); in the tumor DNA (T) the wild-type allele is lost. Panel B: WT1 microsatellite analysis indicating LOH in tumor DNA (T); the two alleles present in the peripheral blood leukocytes (PBL) are indicated (A1 and A2), the lost allele is marked by the asterisk. Panel C: WTX microsatellite analysis indicating LOH in tumor DNA (T); the two alleles present in the peripheral blood leukocytes (PBL) are indicated (A1 and A2), the lost allele is marked by the asterisk. Panel D: HUMARA assay indicating LOH in tumor DNA (T); the two alleles present in the peripheral blood leukocytes (PBL) are indicated (A1 and A2), the lost allele is marked by the asterisk; amplification after digestion of the tumor DNA with methylation sensitive restriction endonucleases (T/d) indicates that the allele retained in the tumor (A2) is digested (arrow), thus, unmethylated, and then on the Xa.
This mutation arose de novo, since both parents were wild type at the same locus.
Analysis of the tumor DNA by direct sequencing of the TSC2 exon bearing the germline mutation, revealed that only the mutant allele was still present in the neoplastic tissue, thus indicating the somatic loss of the wild-type TSC2 allele in the WT (Fig. 2, panel A).
Investigation of polymorphic markers associated to WT1 and WTX disclosed LOH involving both genes in the tumor (Fig. 2, panels B,C). A HUMARA assay performed on not pre-digested tumor DNA showed that the loss involving WTX extended to the AR gene (Fig. 2, panel D), and when the same analysis was performed on tumor DNA digested with methylation-sensitive restriction endonucleases prior to PCR amplification, a marked reduction of the signal of the retained allele was observed, consistently with the DNA being subject to enzymatic digestion and, therefore, unmethylated (Fig. 2, panel D). Since methylation of the HUMARA locus correlates with X chromosome inactivation [Allen et al., 1992], this finding indicates that in the tumor the retained allele was localized on the Xa and the deletion involving both WTX and AR occurred on the Xi.
Sequencing analyses revealed no mutations involving the remaining WT1 and WTX alleles, or TP53 and CTNNB1 genes.
DISCUSSION
Children with congenital anomalies have a higher age-related risk of developing cancer than the general population [Agha et al., 2005]. Moreover, some congenital disorders are specifically associated with certain tumors types.
TSC is a rare, multisystem, autosomal dominant disorder characterized, among other clinical features, by solid and cystic renal lesions, which include angiomyolipoma, oncocytoma, renal cell carcinoma and, depending on the underlying genetic alteration, polycystic kidney disease [reviewed in Siroky et al., 2009].
We here reported on a girl, affected with TSC, who developed a WT diagnosed at age 14 months.
WT is a malignant embryonal tumor of the kidney affecting 1:10,000 children. While most WT cases are sporadic, it is well known its occurrence in association with certain inherited syndromes, including the WAGR (WT, Aniridia, Genitourinary anomalies, Mental retardation) (OMIM#194072), the Denys-Drash (OMIM #194080), and the Beckwith-Wiedemann (OMIM #130650) syndromes.
WT has very rarely been reported in individuals with other genetic disorders, such as Simpson–Golabi–Behemel syndrome [Hughes-Benzie et al., 1996], Li-Fraumeni syndrome [Hartley et al., 1993; Birch et al. 2001], hyperparathyroid-jaw tumor [Kakinuma et al., 1994], neurofibromatosis [Stay and Vawter, 1997], Bloom syndrome [Cairney et al., 1987], Perlman syndrome [Perlman, 1986], Sotos syndrome [Maldonado et al., 1984], trisomy 18 [Geiser, 1973; Karayalcin et al., 1981], trisomy 13 [Olson et al., 1995], Turner syndrome [Say et al., 1971; Olson et al., 1995; Hasle et al., 1996; Spreafico et al., 2007], and Down syndrome [Kusumakumary et al., 1995; Spreafico et al., 2007], and the Frasier syndrome (OMIM #136680).
To the best of our knowledge, only one case of WT in a patient with TSC was previously published: Grether et al. [1987] described a 20-month-old boy with very marked psychomotor retardation, seizures, fibrous angiomatous lesions on cheeks and forehead, ovoid “white nevi” on the trunk and extremities and retinal phacomas, and normal constitutional karyotype.
Our patient represents the second instance of the association of TSC and WT. Due to the symptoms related to TSC (seizures, cardiac impairment), balancing between chemotherapeutic intensity and the risk of co-morbidity was a real challenge in treating this case.
The data obtained from the molecular characterization of the tumor biopsy suggest that different somatic events likely contributed to the etiology of WT in this girl. In fact, we could identify the loss of the constitutionally wild-type TSC2 allele, an event which occurs with a high frequency in TSC patients, and the loss of one WT1 allele, a genetic lesion which is consistent with WT. The WTX deletion that we identified occurred on the Xi, and so it has no clear biological role in the development of the neoplasia. Pathological examinations of the surgical specimen, indicating the presence of diffuse anaplasia and p53 immunoreactivity also implied TP53 gene alteration in the pathogenesis of the disease in our patient. We could not identify the TP53 gene mutation, but our results were obtained in a biopsy sample not containing anaplastic cells.
The paucity of reports on patients with TSC developing WT suggests that these two conditions are unlikely to be genetically related, and occurred together in the two patients by chance. However, the present molecular data could also indicate that, at least on the genetic background of a TP53 anomaly and loss of a WT1 allele, the complete inactivation of TSC2 could lead to WT development. This may support the interest in the further investigation of the role of TSC genes in the pathogenesis of sporadic WT.
Acknowledgements
This work was partially supported by Italian Association for Cancer Research (AIRC) and Associazione Bianca Garavaglia, Busto Arsizio, Varese, Italy.




