Creatine transporter deficiency in two half-brothers†
How to Cite this Article: Ardon O, Amat di San Filippo C, Salomons GS, Longo N. 2010. Creatine transporter deficiency in two half-brothers. Am J Med Genet Part A 152A:1979–1983.
Abstract
X-linked cerebral creatine deficiency is caused by the deficiency of the creatine transporter encoded by the SLC6A8 gene. Here, we report two half-brothers with this condition and characterize creatine transport in human fibroblasts. The propositus presented at 6 months of age with delays in development and slow progress since then with no regression. Seizures started at 3.5 years of age and responded well to treatment with anticonvulsants. He had failure to thrive with all growth parameters (including head size) at or below the fifth centile. Brain MRI indicated hemispheric white matter abnormalities, while MR spectroscopy indicated markedly reduced creatine peak. Biochemical testing indicated increased urine creatine/creatinine ratio, with normal plasma creatine and guanidinoacetate. To confirm the diagnosis, we measured [14]C-creatine transport in fibroblasts. [14]C-Creatine transport in normal human fibroblasts was linear for up to 2 hr at 37°C. Kinetic studies indicated the presence of a single saturable creatine transporter with a Km of 34.7 ± 2.5 µM. Fibroblasts from the propositus lacked creatine transport. DNA testing indicated hemizygosity for a novel deletion producing a frameshift (c.974_975delCA, p.Thr325SerfsX139) in the creatine transporter gene. His 12-year-old half-brother had similar biochemical and clinical abnormalities except for the presence of macrocephaly and the absence of seizures. The mother had history of seizures in childhood, but had normal development. These results show that human fibroblasts have a single major creatine transporter and that measurement of its specific activity can confirm creatine transporter deficiency. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Creatine is required for the utilization of ATP-derived energy at sites of high-energy utilization (muscle, brain, and heart) [Wallimann et al., 1992; Wyss and Kaddurah-Daouk, 2000]. In humans, creatine is synthesized in the kidney and liver as well as obtained from the diet [Brosnan and Brosnan, 2007]. Creatine and its phosphorylated form, phosphocreatine, spontaneously break down to creatinine that is excreted in the urine [Brosnan and Brosnan, 2007]. Conservation and inter-organ transfer of creatine require specific membrane transporters. The creatine transporter gene SLC6A8 encodes the creatine transporter 1 (CT1 or CRTR), maps to Xq28 and is expressed in most human tissues, with highest levels found in skeletal muscle and kidney [Gregor et al., 1995]. A defect in the CT1 creatine transporter results in brain creatine deficiency (OMIM #300352), an X-linked disorder that in affected hemizygous males is characterized by mental retardation, delays in language and speech, autistic-like behavior, seizures in about 50% of cases, and in some cases midfacial hypoplasia, and short stature [Salomons et al., 2001; Clark et al., 2006]. Affected females can have mild cognitive impairment with behavior and learning problems [Stockler et al., 2007]. To date, more than 21 mutations in the SLC6A8 gene have been reported in patients with varying degrees of mental retardation. The prevalence of SLC6A8 mutations in X-linked mental retardation varies between 1% and 5.4% [Rosenberg et al., 2004; Newmeyer et al., 2005; Clark et al., 2006; Lion-Francois et al., 2006; Arias et al., 2007; Betsalel et al., 2008; Puusepp et al., 2009].
The diagnosis of brain creatine deficiency is based on clinical presentation and abnormal brain magnetic resonance spectroscopy with reduced brain creatine content [Verhoeven et al., 2005]. This diagnosis can be confirmed by measurement of long-term creatine accumulation in fibroblasts or by DNA sequencing [Salomons et al., 2003].
In this report, we describe two half-brothers with X-linked brain creatine deficiency and characterize creatine transport in human fibroblasts.
CLINICAL REPORT
The propositus (II-4, Fig. 1A) presented at 6 months of age with developmental delays. He had failure to thrive with all growth parameters (including head size) at or below the fifth centile. Seizures started at 3.5 years of age and responded well to treatment with anticonvulsants. The 12-years-old half-brother on the mother's side (II-3) was similarly delayed, with macrocephaly, failure to thrive early in life, facial features resembling fragile X syndrome, autism, attention deficit disorder with hyperactivity. The mother had no history of learning disabilities and completed college, but had seizures in college.
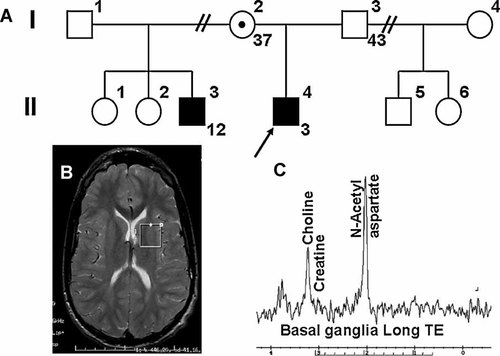
Creatine transporter deficiency: family history and MR studies. A: The two affected half-brothers are indicated with filled symbols in the pedigree. B: Brain MRI of patient II-3 indicated small corpus callosum and diminished white matter in the area of trigone of the lateral ventricle. The voxel sampled for spectroscopy is indicated as a square. C: MR spectroscopy shows a markedly decreased creatine peak.
The propositus (II-4) brain MRI had only minor anomalies with a mild increase in the T2 signal in the hemispheric white matter, particularly in the posterior centrum semiovale, sparing the subcortical and periventricular white matter (not shown). The posterior body of the corpus callosum was mildly thinned. Brain MR spectroscopy indicated markedly decreased creatine in the white matter and in the basal ganglia white matter. Brain MRI in the half-brother, II-3, was abnormal with small corpus callosum and diminished white matter in the area of trigone of the lateral ventricle (Fig. 1B), while MR spectroscopy (Fig. 1C) showed markedly reduced creatine peak.
Biochemical testing in the propositus (II-4) and the half-brother (II-3) indicated normal plasma levels of creatine (103 and 99 µM, respectively, normal 76 ± 22 µM) and guanidinoacetate (1.45 and 1.79 µM, respectively, normal 1.36 ± 0.64 µM). The urine creatine/creatinine ratio was increased in both brothers (4.83 in II-4, normal 0.01–1.19; and 2.03 in II-3, normal for age 0.01–0.4).
MATERIALS AND METHODS
DNA Sequencing
The SLC6A8 gene was sequenced using primers flanking each exon as previously described [Rosenberg et al., 2004].
Biochemical Genetics Testing
Measurement of plasma and urine guanidinoacetate, creatine, and creatinine was performed in CLIA-approved laboratories according to standard procedures.
Cell Culture and Creatine Transport
Fibroblasts from patients and normal controls were cultured in DMEM supplemented with 15% fetal bovine serum and 1% L-glutamine. Creatine transport was measured at 37°C with the cluster-tray method with minor modifications of our previously described method [Amat di San Filippo et al., 2003; Amat di San Filippo and Longo, 2004]. Briefly, cells were grown to confluence in 24-well plates (Costar) and depleted of intracellular amino acids by incubation for 60 min in Earle's balanced salt solution containing 5.5 mM D-glucose and supplemented with 0.5% bovine serum albumin. Cells were then incubated with [14]C-creatine 0.1 µCi/ml (Moravek Biochemicals, Brea, CA) containing the indicated concentration of cold creatine (0.5–2,000 µM) for the time shown. Non-saturable creatine transport was measured in the presence of 2 mM cold substrate. The transport reaction was stopped by rapidly washing the cells four times with ice-cold 0.1 M MgCl2. Intracellular creatine was extracted from the cells with 0.5 ml of ice-cold ethanol and added to 5 ml of scintillation fluid for counting. Intracellular creatine was normalized for the protein content of each well and intracellular water content and expressed as nmol/ml cell water/hr [Amat di San Filippo et al., 2003; Amat di San Filippo and Longo, 2004]. Saturable creatine transport was calculated by subtracting transport in the presence of excess (2 mM) cold substrate from total transport and values are reported as means ± SE of 3–6 independent determinations.
Data were also analyzed using a Michaelis–Menten equation [Amat di San Filippo et al., 2003; Amat di San Filippo and Longo, 2004] after subtraction of non-saturable creatine transport (measured in the presence of 2 mM creatine).
RESULTS
Creatine Transport by Human Fibroblasts
The clinical presentation, MR spectroscopy, and the laboratory results of plasma and urine guanidinoacetate, creatine, and creatinine measurements suggested a defect in the creatine transporter. A skin biopsy was obtained for diagnostic purposes to confirm a defect in cellular creatine transport.
Figure 2 shows the time course for creatine (2 µM) uptake by human fibroblasts. In normal cells, net creatine uptake (total uptake minus the non-saturable uptake measured in the presence of 2 mM unlabeled creatine) was linear for up to 2 hr (r2 = 0.999, P < 0.001 with linear regression of points up to 2 hr) and then declined slightly at the 4 hr time point (panels A and B). Net creatine uptake in cells from the patient was negligible at early time points and was <3% of normal at the longest time points (panels C,D).
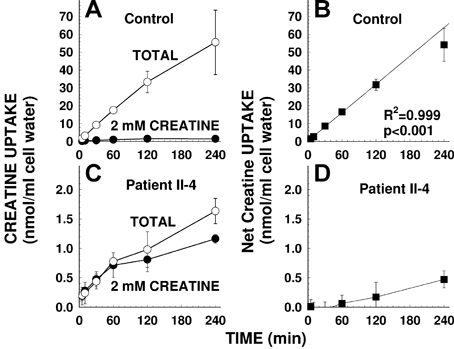
Time-course of creatine transport in human fibroblasts. Creatine (2 µM) uptake was measured for the indicated time in cells from a normal male control (A,B) and from patient II-4 with creatine transporter deficiency (C,D) in the absence (open circles) or presence of excess (2 mM) creatine (closed circles). Net uptake was calculated by subtracting uptake in the presence of excess creatine from total uptake (B,D). Points are averages of triplicates ± SD. The line in panel B represents a linear regression for time points up to 2 hr with the results indicated. Note the different scale in panels A and B as compared to C and D.
Since creatine transport was linear up to 2 hr, we measured the initial rate of creatine (0.5–500 µM) entry for 1 hr in normal fibroblasts (Fig. 3). Creatine transport was compatible with the presence of a saturable system and a linear component formally indistinguishable from diffusion (panel A). Non-linear regression analysis of the data according to Equation (1) (see Materials and Methods Section) indicated a Km of 30.6 ± 3.4 µM toward creatine with a Vmax of 256 ± 12 nmol/ml cell water/hr.
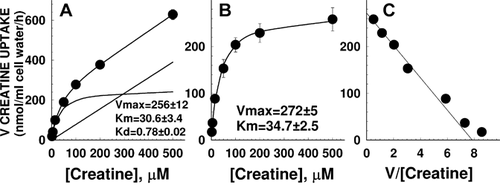
Kinetic constants for creatine transport in human fibroblasts. Creatine (0.5–500 µM) uptake was measured for 60 min in cells from a normal male control. Points are averages of triplicates ± SD. A: Total creatine transport was fitted to Equation (1) (see Materials and Methods Section) for a single saturable transporter with superimposed diffusion. Lines show the best fit to total uptake and the predicted saturable component (hyperbola) and diffusion (straight line). B: Creatine transport was corrected for that measured in the presence of 2 mM cold creatine to correct for diffusion. Data were fitted to a Michaelis–Menten equation. C: Eadie–Hofstee plot of saturable creatine transport in human fibroblasts.
To correct for diffusion, creatine uptake in the presence of 2 mM creatine (non-saturable transport) was subtracted from all points. After this correction, only a saturable component was seen (panel B). Non-linear regression of the data according to a Michaelis–Menten equation indicated a Km of 34.7 ± 2.5 µM and a Vmax of 272 ± 5 nmol/ml cell water/hr. These values were not significantly different from those measured by fitting total, uncorrected creatine uptake for a single saturable system with superimposed diffusion (Eq. 1 in Materials and Methods Section, panel A), indicating that subtraction of non-saturable uptake at 2 mM creatine was an effective way to correct for diffusion. An Eadie–Hofstee plot of the saturable uptake data was linear (panel C), indicating that a single creatine transporter (or multiple creatine transporters with the same Km) were operative in human fibroblasts. The value of Km measured in fibroblasts is similar to the value of 37.3 ± 6.8 µM previously reported with overexpression of the human SLC6A8 cDNA in HEK-293 cells [Straumann et al., 2006]. Comparison of creatine transport among multiple normal cells revealed a relatively narrow range for normal activity, with no significant differences between cells from males and females (Fig. 4). The patient's fibroblasts demonstrated markedly impaired activity that was reduced to 0.2–3% of normal in different experiments.
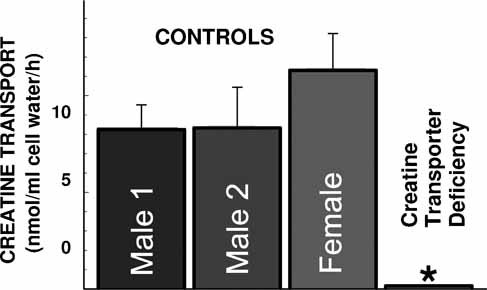
Creatine (2 µM) uptake in human fibroblasts. Creatine transport was measured for 1 hr at 37°C in cells from patient II-4 and 3 unrelated controls. Non-saturable uptake, measured in the presence of excess creatine (2 mM) was subtracted from total uptake to calculate net uptake. Points are averages of multiple experiments (n > 6) performed in triplicates in different days. Data are expressed as average ± SE. Most of the variability was due to day-to-day variation. Creatine transport in cells from patient II-4, however, was always minimal to absent. *P < 0.01 versus controls using analysis of variance.
DNA Sequencing
To further confirm the diagnosis, the SLC6A8 gene encoding the creatine transporter was sequenced in DNA extracted from the propositus's fibroblasts. Patient II-4 was hemizygous for a 2 bp deletion (c.974_975delCA, exon 6), producing a frameshift (p.Thr325SerfsX139). This mutation has not been previously described, but is predicted to abolish function due to the premature insertion of a stop codon.
DISCUSSION
Disorders of creatine synthesis and cellular transport result in brain creatine deficiency and represent a relatively novel cause of mental retardation and seizures. Patients with X-linked creatine transporter deficiency can have different clinical presentations even within the same family [Stockler et al., 2007], with developmental delays/mental retardation being present in all patients. This disease is suspected from reduced brain creatine content detected by MR spectroscopy (Fig. 1). An increased creatine/creatinine ratio in urine further suggests the diagnosis, but there are no abnormal metabolites (such as guanidinoacetate) or low levels of plasma creatine to point to a specific defect. Increased creatine/creatinine ratio in urine can be found in people taking supplemental creatine or on a diet with high-creatine content [Brosnan and Brosnan, 2007]. Therefore, the diagnosis of creatine transporter deficiency must be confirmed by functional studies, by DNA testing or by both [Rosenberg et al., 2007].
Since the first description of creatine transporter deficiency, it was evident that fibroblasts isolated from affected patients failed to accumulate creatine [Salomons et al., 2001; Hahn et al., 2002; Clark et al., 2006]. However, the actual creatine transport activity in these cells has never been measured. Our results show that creatine transport in human fibroblasts is linear for at least 2 hr (Fig. 2). Measurement of kinetic constants for creatine transport indicated the presence of a single saturable transporter with superimposed apparent diffusion (Fig. 3). Correction for diffusion could be accomplished by mathematical means or by subtracting from total transport the activity measured in the presence of excess (2 mM) unlabeled creatine. The apparent Km for creatine transport obtained in fibroblasts (30.6 ± 3.4 and 34.7 ± 2.5 µM) was identical to that previously reported for HEK-293 cells overexpressing the SLC6A8 creatine transporter (37.3 ± 6.8 µM) [Straumann et al., 2006]. Finally, creatine transport activity was absent in cells from a patient hemizygous for a 2 bp deletion resulting in the premature insertion of a stop codon (Figs. 2 and 4). These data indicate that saturable creatine transport in human fibroblasts occurs only through the SLC6A8 transporter.
Comparison of creatine initial rate of entry among different normal controls indicated similar activity in cells obtained from males and females (Fig. 4), which is expected due to X-inactivation in females. Fibroblasts from the patient with a mutation in the creatine transporter gene had a negligible level of creatine transporter activity. These data confirm that fibroblasts can be used to confirm or exclude a diagnosis of creatine transporter deficiency [Rosenberg et al., 2007]. The novel radioactive assay can measure the specific creatine uptake, and thus may be more suitable to detect residual uptake. The assay may also be of value for the determination of the function of SLC6A8C splice variants [Martinez-Munoz et al., 2008]. Therefore, even though phenotypic variability can be observed within family members who are hemizygous for the same SLC6A8 mutation, the availability of this novel functional assay will allow the correlation of residual creatine transport activity with specific mutations and determine whether milder phenotypes could be associated with residual partial activity of the transporter.
Treatment of patients with creatine transporter deficiency remains ineffective [Salomons et al., 2003]. Creatine supplements at standard doses (400–800 mg/kg per day) generate plasma creatine concentrations of about 1 mM. This concentration is much higher than the Km of the creatine transporter (30–35 µM, Fig. 3) and is expected to allow entry of creatine through less specific transporters. However, the presence of physiological substrates of these other transporters in vivo and the need of transferring creatine across the multiple cells types part of the blood–brain barrier are likely to impair substrate transfer to the brain. This is the probable explanation for the lack of any increase in brain creatine levels in our patients (not shown) while on creatine supplements and other therapies. Other therapies, with the creatine precursors arginine and glycine, are more likely to be effective [Chilosi et al., 2008].




