Robinow syndrome: Phenotypic variability in a family with a novel intragenic ROR2 mutation†
How to cite this article: Brunetti-Pierri N, del Gaudio D, Peters H, Justino H, Ott C-E, Mundlos S, Bacino CA. 2008. Robinow syndrome: Phenotypic variability in a family with a novel intragenic ROR2 mutation. Am J Med Genet Part A.
Abstract
Robinow syndrome comprises dysmorphic facial features, short stature, brachymesomelia, segmental spine defects, and genital hypoplasia. The range of severity in this disorder is broad. We report on the clinical and molecular findings of two sib pairs from the same extended family with Robinow syndrome due to a novel intragenic ROR2 deletion involving exons 6 and 7 that could not be detected by sequencing. The affected individuals exhibited variability with respect to the cleft lip, cleft palate, and cardiac findings and for the presence in one of the patients of syringomyelia, which has not been previously reported in Robinow syndrome. © 2008 Wiley-Liss, Inc.
INTRODUCTION
The autosomal recessive form of Robinow syndrome (OMIM 268310) comprises short stature, brachymesomelia, segmental spine defects, and genital hypoplasia. As initially described by Robinow et al. 1969, the facial appearance, at least in young patients [Soliman et al., 1998; Tufan et al., 2005], resembles a fetal face with relatively small face, hypertelorism, wide palpebral fissures, broad nasal base, everted nares, and broad and triangular mouth. Robinow syndrome can be caused by homozygous or compound heterozygous loss of function mutations in ROR2, an orphan receptor tyrosine kinase [Afzal et al., 2000; van Bokhoven et al., 2000; Chen et al., 2005].
CLINICAL REPORTS
The research was approved by the Baylor College of Medicine Institutional Review Board. The affected individuals comprise two sibling pairs that were evaluated in two different institutions, several years apart (Fig. 1). All four parents of both sib pairs were healthy and had no skeletal or hand abnormalities on clinical examination.
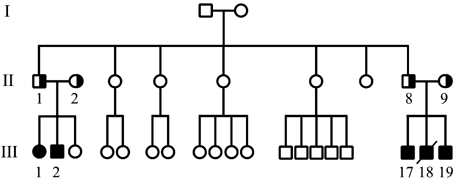
Pedigree of the presented family.
Affected Individual III.1
This patient was born after 40 weeks from an unremarkable gestation with a weight of 1,690 g (<10th centile). She had a cleft lip and palate that required multiple reconstructive procedures. Her development milestones and school performance were normal. She had normal pubertal development, menarche at 11 years of age, and regular menstrual cycles. Physical examination at age 18 years showed OFC of 58.5 cm (∼+2 SD), a height of 130 cm (≪5th centile), and a weight of 41 kg (≪5th centile). She had upslanting palpebral fissures, hypertelorism, a broad nose (Fig. 2A), marked scoliosis, mesomelic shortening, limited pronation and supination of the forearms, brachydactyly, fusion of the F3,4 on the right, T1 was broad and fusion of T2,3, absence of the T4 on the right foot, fusion of the T1,2 of the left, and small nails (Fig. 2A). The skeletal survey showed an S-shaped scoliosis with vertebral fusions and hemivertebrae. Radiographically, she had osseous syndactyly of the right proximal phalanges of F3,4, small distal and middle phalanges of F2-5. The left hand showed small middle and distal phalanges and fusion of the middle and distal phalanges of F4,5. The ulnae were short with distortion of the distal articular surface. At the age of 17 years, she developed back pain and a spine MRI showed thoracic syringomyelia (Fig. 3), which was treated with a thoracic laminectomy and a syringo-subarachnoid shunt.
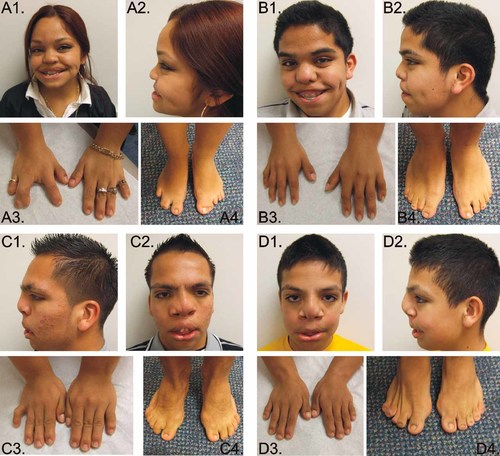
Affected individual III.1 at the age 18 years (A1–A4); affected individual III.2 at the age of 17 years (B1–B4); affected individual III.17 at the age of 17 years (C1–C4), cleft lip repair scar is noted; affected individual III.19 at the age of 12 years (D1–D4), signs of previously corrected cleft lip and palate can be noted. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
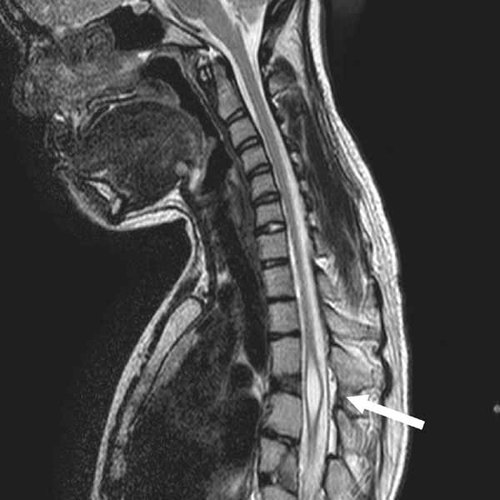
Spine MRI showing a focal syrinx from T4 to T7 (arrow) in the spinal canal (individual III.1).
Affected Individual III.2
This patient presented in the newborn period with micropenis, hypospadias, and right cryptorchidism. Birth measurements are unknown. In contrast with his sister (III.1), he had no cleft lip and palate but he had a high-arched palate. He had normal developmental milestones. On physical examination at age 17 years his weight was 45.9 kg (<5th centile), height was 136.1 cm (≪5th centile), and his head circumference was 57.2 cm (0 to +2 SD). He had up-slanting palpebral fissures, hypertelorism, broad nasal tip, short philtrum, and large mouth (Fig. 2B), pectus excavatum, mesomelic shortening of the upper limbs, and short hands (right hand length 13.4 cm and the left 14.5 cm, both <3rd centile). Genital exam showed a micropenis with testes of normal adult size with Tanner IV development. The skeletal survey revealed hemivertebrae and fusions, 11 ribs on the right and 10 on the left, and partial fusion of the posterior left seventh through tenth ribs. On radiological exam, the arms were remarkable for shortening of the ulnae, and absence of the styloids bilaterally. The first metacarpal and the middle and distal phalanges of F2-4 were short. On the feet, fusion of the middle and distal phalanges of T3-5 on the left and T2-4 on the right were noted. The echocardiogram showed a bicuspid aortic valve and a coarctation of the aorta. The cardiac MRI confirmed the coarctation of the aorta distal to the left subclavian artery, in the juxta-ductal area and narrowing of the aortic isthmus (Fig. 4A,B).
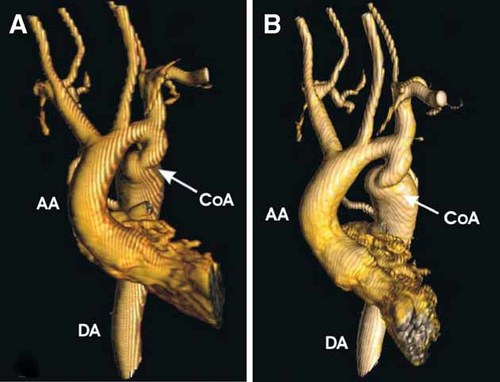
Three-dimensional magnetic resonance angiography of coarctation of the aorta in frontal projection (A) and left anterior oblique projection (B) (individual III.2). The coarctation was extremely tortuous, and the narrowest diameter was approximately 9 mm, with post-stenotic dilatation of the entire descending thoracic aorta, to a maximal diameter of approximately 32 mm. AA = ascending aorta, DA = descending aorta, CoA = coarctation (Courtesy of Dr. Timothy C. Slesnick, Texas Children's Hospital). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Affected Individual III.17
His gestational and birth history are unknown. He had cleft lip and palate and genital hypoplasia at birth. His gross and fine motor milestones were normal, although he had mild delays in language acquisition. At the age 17 years his height was 142.7 cm (<5th centile) and his weight 59.6 kg (10th–25th centile). He had hypertelorism, broad nasal bridge with bifid nose, and dental crowding (Fig. 2C), mesomelic shortening of the upper limbs, limited pronation and supination of the forearms, and short hands (right hand 15 cm and the left 14 cm, both <3rd centile) (Fig. 2C). The penis was small, but both testes were of normal adult size and Tanner stage IV. The skeletal survey revealed scoliosis and multiple vertebral anomalies including hemivertebrae and multiple fusions, rib fusions, shortening of the ulna and radius.
Affected Individual III.18
This baby also has an unknown gestational and birth history, but died following an episode of fever at age 2 months. According to the history he had a cleft lip, cleft palate and ambiguous genitalia. His gender assignment was male.
Affected Individual III.19
He was born at term to an uncomplicated gestation by cesarean section performed because of transverse lie. His birth parameters are unknown. He had bilateral cleft lip and palate at birth. He had problems with frequent lacrimation secondary to obstruction/atresia of the lacrimal ducts bilaterally. His developmental milestones were normal. At the age of 12 years, his weight was 41.1 kg (25th–50th centile), his height 136.2 cm (<5th centile) and his OFC was 54.9 cm (∼75th centile). On examination he had hypertelorism, broad nasal bridge, and surgical scars from his corrected cleft lip and palate, acromesomelic shortening of the upper limbs, limited pronation and supination of the forearms, and brachydactyly (right hand 13 cm and the left hand 13 cm, both <3rd centile) (Fig. 2D). Both testes were adult size, pubic hair was Tanner stage III, and he had a micropenis. The skeletal survey showed hemivertebrae, vertebral fusions, and shortening of the forearms.
METHODS
ROR2 Mutation Analysis
Genomic DNA from peripheral blood leukocytes was extracted using standard protocols. PCR primers were designed for amplification of all coding exons and flanking intronic sequences of ROR2 (RefSeq NM_004560.2) and are available upon request. PCR products were sequenced using standard methods on an ABI Prism 3730 XL Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequence analysis and assembly were performed using the Mutation Surveyor software V3.2 (SoftGenetics, State College, PA).
Quantitative Real-Time PCR (qPCR)
To evaluate genomic DNA for the ROR2 exons 6 and 7 deletion we used a qPCR test, using four sets of primer pairs located within the deletion (supporting information Table I may be found in the online version of this article). qPCR was performed as previously reported [Klopocki et al., 2007] and samples were run in triplicate in separate reactions to permit the quantification of the target sequences normalized to ALBUMIN (ALB). PCR conditions were as follows: initial denaturation step at 95°C followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. By use of calibrator samples of normal control DNA, the gene copy number was estimated on the basis of the comparative ΔΔCt method [Livak and Schmittgen, 2001]. In addition, we performed a sex determination for the individuals, calculating the Factor VIII exon 3 relative to our endogenous control ALB, to assure its reliability.
Long-Range PCR Amplification and Deletion Breakpoint Analysis
Long-range PCR was performed using a forward primer in ROR2 exon 5 and a reverse primer in exon 8 (ROR2 Ex5F 5′-AGTAACAAAATTGCTGGATCGCA-3′ and ROR2 Ex8 R 5′-AAGACATTTAATGTTGGGGGAAAC-3′) (Expand Long Template Enzyme mix; Roche Diagnostics, Basel, Switzerland). Nested sequencing primers were designed for exact identification of deletion breakpoints (forward primer: 5′-GACTTAACTTCTCTGATTGGCTGTC-3′; reverse primer: 5′-AAAGCAATTGAATGTAAGACAAAGTG-3′) and PCR products were sequenced as previously described.
RESULTS
ROR2 Mutation Analysis and qPCR
No mutations were found in ROR2 exons 1–5, 8, and 9. Exons 6 and 7 could not be amplified in the affected individuals, but were amplified in the unaffected parents and control DNA (data not shown), suggesting that the patients had a homozygous deletion of ROR2 exons 6 and 7. Copy number analysis of ROR2 exons 6 and 7 was performed on the affected brothers (III.17, III.19) and their unaffected parents (II.8 and II.9) by qPCR to evaluate the deletion carrier status. Both parents had the intragenic deletion of the ROR2 exons 6 and 7 in a heterozygous state while no amplification was detectable in the two affected brothers (Fig. 5A). The two brothers were homozygous for the 85 bp junction fragment between intron 5 and intron 7 whereas their parents had one copy of this fragment and there was no amplification in the qPCR control sample (Fig. 5A).
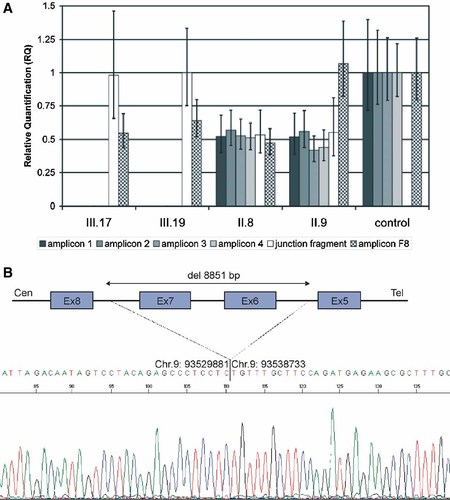
A: Copy number analysis by qPCR for affected siblings (subjects III.17 and III.19) and carrier parents (II.8 and II.9). Amplicon 1-4 (gray bars) and amplicon F8 (checkered bars) results were calibrated to the mean value determined for a healthy female control. The 85 bp junction fragment values (white bars) were calibrated to the mean value of sample III.19. Error bars indicate the 95% confidence interval as given by the ABI 7500 SDS-Software. B: Long-range PCR amplification and deletion breakpoint analysis. Nested sequencing analysis performed on the long range PCR junction fragment showed a deletion encompassing 8,851 bp with the 3′ end mapping in ROR2 intron 5 and the 5′ end mapping in ROR2 intron 7. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Long-Range PCR Amplification and Deletion Breakpoint Analysis
In the patients, long range PCR with ROR 2 exon 5 forward and exon 8 reverse primers resulted in a product of about 2 kb, while no PCR product was obtained in controls. Nested sequencing of the patient's PCR junction fragment revealed a 8,851 bp deletion with the 3′ end in ROR2 intron 5 and the 5′ end in ROR2 intron 7 (Fig. 5B). We concluded that the centromeric breakpoint of the intragenic deletion to be between nucleotides 93,529,881 and 93,529,882 on chromosome 9 and the telomeric between nucleotides 93,538,732 and 93,538,733, respectively (Ensembl release 49, March 2008). This deletion and the breakpoints were confirmed in all affected members of the family.
DISCUSSION
Fifteen distinct deletion, missense, nonsense, and frameshift mutations have been reported in patients with Robinow syndrome in various domains of the ROR2 protein including the frizzled-like, kringle, tyrosine kinase, and Ig-like domains [Afzal et al., 2000; van Bokhoven et al., 2000; Tufan et al., 2005; Ali et al., 2007]. In two sib pairs from an extended family affected with Robinow syndrome, we report on a novel deletion encompassing a ∼8.8 kb fragment between ROR2 introns 5 and 7. As the end phase in exon 5 and the start phase in exon 8 are identical, the mRNA splicing might result in an in frame product lacking a major part of the frizzled-like domain, but we did not perform experiments to test for the mutant mRNA. This domain may be crucial for stable folding and proper post-translational modification of ROR2, and mutations in this domain have been shown to cause endoplasmic reticulum protein degradation [Ali et al., 2007]. Due to the large size, the mutation could also induce nonsense mediated mRNA decay. We conclude that this mutation most likely leads to loss of function of ROR2.
Neurologic manifestations are not a typical component of Robinow syndrome. Mental retardation, developmental brain anomalies, communicating hydrocephalus, and seizures have been reported in a small number of cases [Saal et al., 1988; Guillen-Navarro et al., 1997; McPherson et al., 2006]. Syringomyelia has not been reported to our knowledge. Syringomyelia has been reported in association with certain congenital anomalies or in other genetic conditions including Williams syndrome [Cohen and Quigley, 2006], cleidocranial dysplasia [Dore et al., 1987], Crouzon syndrome [Cinalli et al., 1995], von-Hippel-Lindau syndrome [Probst et al., 1978] mucopolysaccharidosis type VI [Hite et al., 1997], Nager syndrome [Groeper et al., 2002], Carpenter syndrome [Islek et al., 1998], and Dandy-Walker malformation [Hammond et al., 2002]. Syrinxes are often associated with scoliosis [Kuntz et al., 2004], and in many of cases are due to Chiari I malformation [Ghanem et al., 1997]. The pathogenesis of syrinx formation is unknown.
In conclusion, we report on the clinical and molecular findings of two pairs of siblings from the same extended family affected with Robinow syndrome due to a novel intragenic ROR2 deletion. The clinical presentation in these patients demonstrates intrafamilial variability with respect to the cleft lip, cleft palate, and cardiac abnormalities. Whether the syringomyelia is caused by the ROR2 mutation awaits confirmation in other cases, as it may be coincidental.




