A small and active ring x chromosome in a female with features of Kabuki syndrome†
How to cite this article: Rodríguez L, Diego-Alvarez D, Lorda-Sanchez I, Gallardo FL, Martínez-Fernández ML, Arroyo-Muñoz ME, Martínez-Frías ML. 2008. A small and active ring X chromosome in a female with features of Kabuki syndrome. Am J Med Genet Part A.
Abstract
A ring X chromosome is found in about 6% of patients with Turner syndrome (TS), often with mosaicism for a 45,X cell line. Patients with this karyotype are reported to have a higher incidence of a more severe phenotype including mental retardation. In fact, some studies have shown a correlation between this severity and the presence or absence of an intact and functional X inactivation center (XIST). However, the phenotype of the individuals with r(X) cannot be entirely defined in terms of their X-inactivation patterns. Nevertheless, a small group of these patients have been described to manifest clinical features reminiscent of the Kabuki syndrome. Here we present a female patient with clinical features resembling Kabuki syndrome and a mos 45,X/46,X,r(X) karyotype. Methylation analyses of polymorphic alleles of the androgen receptor gene showed that both alleles were unmethylated suggesting an active ring chromosome. A specific X chromosome array CGH was performed estimating the size of the ring to be 17 Mb, lacking the XIST gene, and including some genes with possible implications in the phenotype of the patient. © 2008 Wiley-Liss, Inc.
INTRODUCTION
X-chromosome inactivation (XCI) is the mechanism by which mammals compensate the genetic dosage imbalance arising by the different numbers of gene-rich X-chromosomes between females and males [Ng et al., 2007]. Just after fertilization in the pre-implantation female embryos, both the maternal and paternally inherited X chromosomes are active during just a few days. Afterwards, the “switching off” process, also called Lyonization [Lyon, 1962] occurs sequentially in development, coupled with cell differentiation. This process is usually completely random, and once established is clonally maintained; rarely a spontaneous or unprogrammed reactivation occurs. Consequently, the relative proportion of cells with an active maternal or paternal X chromosome varies from female to female. However, when a structurally abnormal X chromosome exists, it will not undergo inactivation when derived from a balanced X-autosome translocation, in order to leave expression of all autosomic genes, and consequently the XCI is “skewed” [Stankiewicz et al., 2006]. Similarly, when an X chromosome is structurally abnormal (duplicated, deleted, iso or ring chromosome), it is usually inactivated in the majority of cells, giving rise to a female phenotype similar to those with a true X monosomy or Turner syndrome (TS) phenotype.
The XCI is initiated from the X-inactivation center (Xic), at Xq13.2, when the XIST gene is expressed. Once initiated, the inactivation spreads from the Xic in both directions along the entire chromosome, affecting most genes located on the X chromosome. Nevertheless, it has been shown that several genes escape inactivation, although the specific mechanism is still unknown. In fact, the TS phenotype, from patients with a 45,X karyotype, is thought to be due either to haploinsufficiency of genes that are among those on the X chromosome that escape X-inactivation and hence, would be expected to be expressed in normal females from both X chromosomes, or to the presence of only one X at the beginning of the embryonic development when the two X chromosomes need to be active for normal female development. The TS phenotype is typically associated with a 45,X karyotype; however, a ring X chromosome is found in about 6% of patients with TS, often with mosaicism for a 45,X cell line [Sybert and Mccauley, 2004]. Some patients have been reported having such mosaicism with a more severe phenotype [Kushnick et al., 1987; Van Dyke et al., 1992; Dennis et al., 1993; Migeon et al., 1993, 1994, 2000] which seems to be associated with the presence or absence of an intact and functional X inactivation center (XIST) [Lepping et al., 2004]. However, the phenotype of the individuals with r(X) cannot be entirely defined in terms of their X-inactivation patterns [Lepping et al., 2004]. In addition, a small group of these patients have been described with clinical manifestations resembling Kabuki syndrome (KS) [Niikawa et al., 1988; Grompe et al., 1992; Kajii et al., 1992; Wellesley and Slaney, 1994; Wolff et al., 1994; Jani et al., 1995; McGinniss et al., 1997; Stankiewicz et al., 2001], a rare congenital disorder of unknown etiology (OMIM # 147920).
Here we report on a female patient with features resembling Kabuki syndrome and a small and active paternally derived X ring chromosome, lacking the XIST gene. A specific X chromosome array CGH was performed to estimate the size of the ring and the possible genes implicated in the phenotype of the patient.
CLINICAL REPORT
The patient was the first daughter of a young (30-year-old mother and 32-year-old father), healthy and non-consanguineous couple. During pregnancy, the fetal measurements were concordant with the gestational ages until the 32nd week, when abnormal short femurs were observed.
The delivery occurred spontaneously at 38 weeks. At birth, she weighted 2,820 g (25th centile), had a length of 45 cm (25th centile) and an occipital-frontal circumference (OFC) of 33.5 cm (50th centile). Her exam showed craniofacial anomalies, with coarse facial features, downslanting palpebral fissures, wide fontanelle, redundant skin in the upper eyelid, wide nasal root, big mouth with downturned corners, thin upper lip with long philtrum, high-arched palate and an oral frenula that produces a notch in the alveolar ridge (Fig. 1a,b). She also had lymphedema of the hands and feet and toes with hypoplastic nails and hypoplastic labia majora with prominent clitoris.
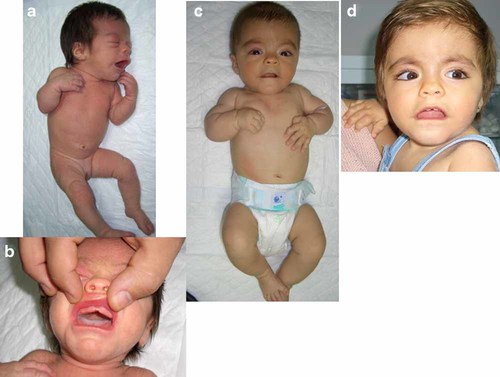
a: Clinical appearance of the patient at birth. b: Detail of the notch in the alveolar ridge. c: The patient at 4 months, with apparently shortened limbs. d: Clinical appearance of the patient at 18 months of age. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.].
At age 4 months, she had apparent shortened limbs (Fig. 1c), but the X-ray was normal. At 18 months, she had a significant growth retardation with a length of 68 cm (-4.6DS) and a slight psychomotor delay. The MRI showed corpus callosum agenesis (CCA).
Figure 1d shows the girl when she was 18 months old with craniofacial anomalies resembling KS patients, including downslanting palpebral fissures, wide nasal root, high-arched palate and the eversion of the lateral part of the lower lid, as well as a significant post-natal growth retardation and psychomotor delay. Figure 2 a–e shows different details of the patient at 3.5 years old.
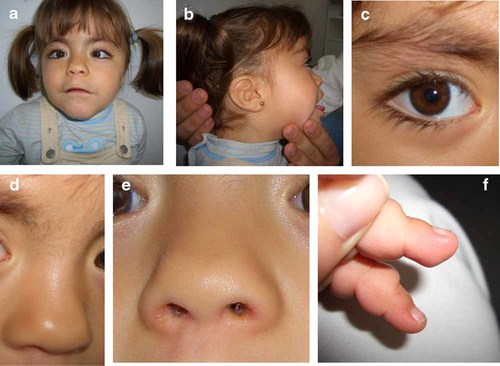
a: Facial appearance of the patient at age 3.5 years. b: Large prominent ears. c: Eversion of the lower lateral eyelid. d: Sparse eyebrows. e: Depressed nasal tip. f: Finger pads. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.].
CYTOGENETIC AND MOLECULAR STUDIES
Cytogenetics analysis of 30 G-band metaphases (550–850 bands) from peripheral blood lymphocytes prepared according to standard procedures, revealed 45,X karyotype in two cells, and 46 chromosomes with absence of one X chromosome and the presence of a small ring chromosome in 28 cells (Fig. 3a). This alteration was “de novo” since the karyotypes of both parents were normal. Fluorescence in situ hybridization (FISH) analyses using the specific centromeric X chromosome probe DXZ1 (Vysis, Inc., Downers Grove, IL), showed two signals in the cells where the ring was present, one in the normal chromosome X and another in the ring chromosome (Fig. 3b), indicating that the ring was derived from the X chromosome. Therefore final karyotype was: mos 45,X [2]/46,X,+mar. ish der(X) (DXZ1+) [28]dn.
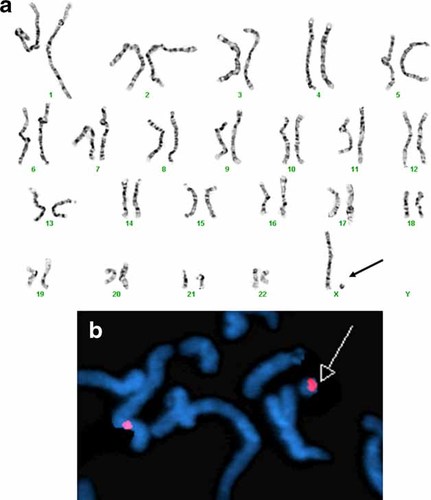
a: High resolution G-band karyotype (550–850 bands) of a cell showing 46 chromosomes: with an absence chromosome X and a small ring chromosome. b: FISH with the specific centromeric X chromosome probe DXZ1, showing two signals, one in the normal chromosome X and another one, in the small ring chromosome. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.].
Further polymorphic microsatellite analysis revealed the paternal origin of the ring X chromosome. A methylation-specific PCR assay was also performed at the CpG island (exon 1) of the AR (androgen receptor) gene (Xq11–q12), where the methylation status correlates with X inactivation, and the results showed an absence of two methylated alleles at this locus, indicating that both were active (Fig. 4).
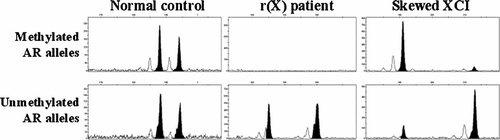
A Methylation-specific PCR assay at the CpG island (exon 1) of the AR gene (Xq11–q12), where methylation status correlates with X inactivation showing the presence of two distinct unmethylated alleles at this locus.
In order to determine the size and gene content of the ring and to exclude subtle imbalances, a whole genome CGH-array was performed [CGH-SANGER 3K6 VIB MAF (containing 3,587 distinct BAC and PAC covering the whole genome with a resolution of 1Mb)], showing no alterations other than the ring X chromosome. A specific X chromosome CGH-array was also performed [CGH-X 1K 1 CGH-X 1K-1 VIB MAF (containing 2,024 different BAC and PAC sequences, being 1,865 specific from the X-chromosome and the rest autosomal ones, with 0.08 Mb resolution)] estimating the size of the ring X chromosome to be of 16.9 Mb (54,845,949–71,764,933 X chromosome nucleotide positions on the genome browser), from clone RP13-391G2 to clone RP11-218L14 (Fig. 5).
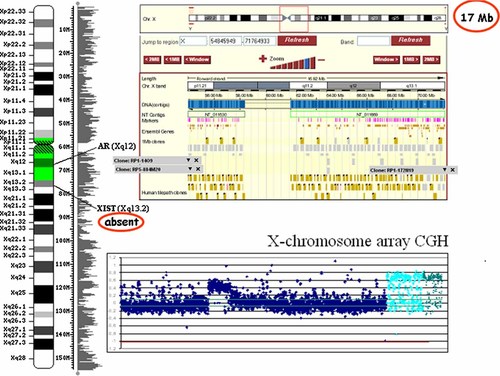
Specific X-chromosome CGH-array revealing the size of the ring X chromosome in about 17 Mb and revealing the absence of the XIST gene from the ring. Dark blue dots higher than the rest represent the (mosaic) X-ring clones content of about 16.9 Mb; dispersed higher dots correspond to polymorphic ones. Sky-blue dots represent autosomal clones and dark-green ones, additional clones lacking a known genome position (data from Ensembl release 44). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.].
DISCUSSION
Most patients with 45,X/46,X,r(X) karyotype have a phenotype resembling the TS phenotype with short stature and premature ovarian failure. Nevertheless, some patients with such karyotype have been described having a phenotype different from TS and with clinical manifestations reminiscent of KS [Grompe et al., 1992; Kajii et al., 1992; Wellesley and Slaney, 1994; Wolff et al., 1994; Jani et al., 1995; McGinniss et al., 1997; Stankiewicz et al., 2001]. This phenotype was interpreted as due to the loss of the XIST gene at Xq13.2, with the subsequent lack of r(X) inactivation, and the functional disomy of the genes contained in the r(X). However, it seems also evident that the size of the r(X) is related to the fact that inactivation happens correctly, as was proposed by Stankiewicz et al. 2001. These authors described a patient having a classical KS phenotype with a small r(X) with XIST expression and proposed that the small r(X) had been unable to undergo a correct inactivation.
The KS, known as Kabuki make-up syndrome or Niikawa–Kuroki syndrome (OMIM # 147920), was described by Niikawa et al. 1981 and Kuroki et al. 1981 and is a pediatric congenital disorder of unknown cause, characterized by mental retardation, post-natal short stature, distinctive features with long palpebral fissures, eversion of the lateral third of the lower eyelids, long eyelashes, broad and depressed nasal tip, large prominent earlobes and cleft, or high-arched palate [Niikawa et al., 1988; Schrander-Stumpel et al., 1994]. Additional findings include skeletal abnormalities, persistence of finger pads, congenital heart defects, urinary tract anomalies, hypotonia, sensorineural hearing loss, and recurrent infections [Adam and Hudgins, 2004].
The patient presented here, has craniofacial anomalies resembling KS patients, including the eversion of the lateral part of the lower lid, as well as significant post-natal growth retardation and psychomotor delay. Her karyotype showed a mosaicism for a 17-Mb r(X), with euchromatic material from Xp11.21 to Xq13.1, lacking the XIST gene and consequently active. Therefore, she had functional disomy of the contained genes on the r(X), which may be responsible of her phenotype. In fact, at Xq pericentromeric region, there is the immunoglobulin-binding protein 1 gene (IGBP1), associated with CCA, with mental retardation, ocular coloboma and micrognathia in an X-linked recessive manner [Abd et al., 1997; Graham et al., 2003]. The female patient described in this report has CCA, which may also indicated a relation among the functional disomy of the IGBP1 gene, and the subsequent high levels of the alpha 4 protein, and the CCA. Nevertheless, ongoing studies on quantification are essentials to clarify the relation among alpha 4 levels and specific clinical manifestations.
With this case we would support the previously reported association between r(X) and the KS phenotype, and to show the challenges for correct genetic counseling once a r(X) is detected in a karyotype. Theoretically, it is widely known that a r(X) should be inactivated and associated with TS phenotype, but it is not that simple. The phenotype associated with r(X), will be only partially predicted depending on the size of the ring, the grade of mosaicism, the presence of the XIST gene and the complete or incomplete status of inactivation of the r(X). Therefore molecular techniques are indispensable for a complete definition of the r(X) content in an effort to perform the best approximation of its clinical effects.
Acknowledgements
We thank the family and their physicians for their collaboration in this study. This work was supported by a Grant (PI020028) from the Fondo de Investigaciones Sanitarias (FIS), Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo, Spain.




