Type II autosomal recessive cutis laxa: Report of another patient and molecular studies concerning three candidate genes†
How to cite this article: Scherrer DZ, Alexandrino F, Cintra ML, Sartorato EL, Steiner CE. 2008. Type II autosomal recessive cutis laxa: Report of another patient and molecular studies concerning three candidate genes. Am J Med Genet Part A.
Abstract
Cutis laxa is a rare disorder of connective tissue in which the skin sags excessively, giving the individual an aged appearance. In the present study we analyzed three unrelated families with type II autosomal recessive cutis laxa for mutations in three genes implicated in other forms of cutis laxa; LOX, FBLN4, and FBLN5 genes. Two individuals have been previously reported, and the third case is described in detail. No causative mutations were identified. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Cutis laxa occurs in acquired and inherited skin diseases and is characterized by pendulous, redundant, and inelastic skin. There is genetic heterogeneity and autosomal dominant, X-linked recessive and three autosomal recessive (ARCL) forms are described [Fitzsimmons et al., 1985; Der Kaloustian, 1993; Imaizumi et al., 1994].
ARCL type II, also called cutis laxa with growth and developmental delay or cutis laxa with bone dystrophy (OMIM 219200), is a rare disorder characterized by pre- and postnatal growth deficiency, mental retardation of mild to moderate degree, large fontanels, hip dislocation, Wormian bones, dysmorphic facial features, scoliosis and osteoporosis [Agha et al., 1978; Allanson et al., 1986; Patton et al., 1987; Imaizumi et al., 1994; Steiner et al., 2005]. Histological findings include aggregation, fragmentation and clumping of elastic fibers [Sakati et al., 1983; Steiner et al., 2005]. It shares many similarities with ARCL type I, which also has histological abnormalities involving the elastic fibers. There is debate as to whether ARCL type II is the same condition as reports of the “wrinkly skin syndrome” and gerodermia osteodysplastica, but this will remain unclear until the molecular basis is understood [Zlotogora, 1999; Al-Gazali et al., 2001; Steiner et al., 2005; Gupta and Phadke, 2006; Boente et al., 2006].
Candidate genes for ARCL type II include genes which code for fibulins, a recently recognized family of extracellular matrix proteins with six known members. These molecules contain series of calcium-binding epidermal growth factor (cbEGF) modules and a C-terminal fibulin domain. Fibulins interact with a multitude of extracellular matrix proteins, including components of basement membranes and elastic fibers [Argraves et al., 2003; Timpl et al., 2003], which are abnormal in individuals with cutis laxa. Loeys et al. 2002 identified a homozygous missense mutation in the FBLN5 gene (EVEC or DANCE) resulting in a serine-to-proline (Ser227Pro) substitution in a large consanguineous Turkish family with ARCL type I. More recently, Hucthagowder et al. 2006 identified mutations in fibulin 4 (FBLN4, EFEMP2 or UPH1) in a patient with ARCL. Although the authors did not specify which form, the clinical description of the patient is compatible with type I.
Another potential candidate is LOX, coding for lysyl oxidase, a key enzyme that controls the processing, cross-linking and maturation of collagen/reticulin and elastin fibers. These cross-links in collagen are essential for the mechanical stability and elasticity of the fibers [Hofer et al., 2004]. As yet mutations in LOX, have not been identified in human disorders but decreased expression levels of lysyl oxidase have been demonstrated in Menkes syndrome and cutis laxa [Khakoo et al., 1997].
Given the rarity of autosomal recessive forms of cutis laxa and the overlap between ARCL type II and other cutis laxa syndromes, we felt it was warranted to look for mutations in candidate genes in three unrelated individuals seen in our service with typical ARCL type II.
MATERIALS AND METHODS
Patients
The present study analyzed DNA from three unrelated patients, two males and a female, presenting with ARCL type II for mutations in LOX, FBLN4 and FBLN5 genes. Patients 1 and 2 have been previously reported in the literature [Steiner et al., 2005], and Patient 3 is described below.
He was born at term after an uneventful pregnancy and delivery, the only living child of healthy nonconsanguineous parents of Portuguese, Afro-Brazilian and Amerindian ancestry. The couple had a previous miscarriage and the family history was otherwise unremarkable. His birth weight was 2,400 g and the birth length 48 cm. In the neonatal period he was noted to have congenital hip dislocation and a senile facial appearance.
A diagnosis of the neonatal progeroid syndrome, Wiedemann–Rautenstrach syndrome was considered. However in the first year of life he showed considerable psychomotor delay, starting language development after the age of two and still unable to walk at age five. He attends a special school. At age 4 years he weighed 9.0 kg and was 85 cm tall with an OFC measuring 45.5 cm (all markedly below the 3rd centile). On examination he had a high forehead with delayed closure of the fontanelles, a triangular face with malar hypoplasia and prognathism (Fig. 1), prominent ears, blue sclera, small and brittle teeth, camptodactyly of the 4th left finger, and a retractile left testis. There was excessive skin with increased wrinkling over the trunk and extremities (Figs. 1 and 2) and visible superficial blood vessels with scanty subcutaneous tissue. There was no abnormal scarring or hyperextensibility that might suggest Ehlers–Danlos syndrome. Investigations included a normal male karyotype, a normal echocardiogram and normal ophthalmologic evaluation. Serum copper was 52.2 µg/dl (normal values: 80.0–160.0 µg/dl) and serum ceruloplasmin was 15.5 mg/dl (normal values: 22.0–58.0 mg/dl). Skeletal survey revealed diffuse osteopenia and bilateral hip dislocation, without visible occipital horns.
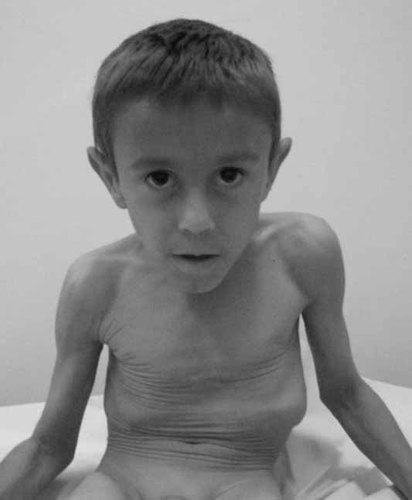
Individual 3 at age 6 years. Note triangular face with malar hypoplasia and prognathism, prominent ears, and increased wrinkling over the abdomen.
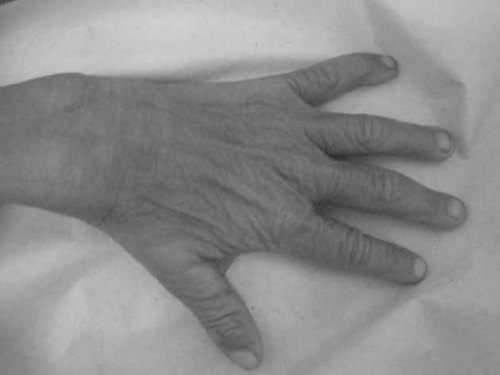
Dorsal view of Patient 3 hands showing loose skin and aged appearance.
A skin biopsy on Weigert-van-Gieson stained sections and on low power ultrastructural view was compared to an age- and site-matched control and showed a decreased amount and irregular distribution of elastic fibers (Fig. 3A). Fragmentation and agglutination of the microfibrillar component and decreased elastin were also seen (Fig. 3B).
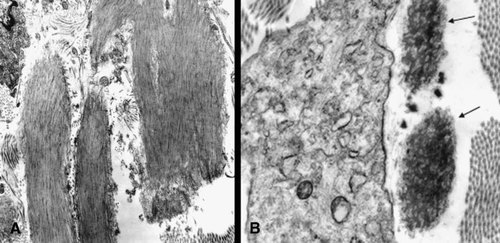
Cutis laxa, torso skin (B); age- and site-matched control skin (A). Fragmentation and agglutination of the microfibrillar component and decreased elastin is seen (arrows) (transmission electron microscopy, original magnification 12,500×).
Table I summarizes clinical findings of Patients 1–3 and compares to the different forms of cutis laxa, wrinkly skin syndrome and gerodermia osteodysplastica.
| Clinical finding | CL | WSS | GO | Patient 1 | Patient 2 | Patient 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| AD | AR I | AR II | AR III | XR | ||||||
| Gender | M | F | M | |||||||
| Onset | Late | Birth | Birth | Birth | Birth | Birth | Birth | Birth | Birth | Birth |
| Intrauterine growth retardation | − | + | + | + | − | + | + | + | + | + |
| Postnatal growth deficiency | − | + | + | + | − | + | + | + | + | + |
| Neuromotor retardation | − | + | + | + | − | + | + | + | + | + |
| Senil appearance | + | + | + | + | + | + | + | + | + | + |
| Loose skin | + | + | + | + | + | + | + | + | + | + |
| Craniofacial dysmorphisms | − | + | + | + | − | + | + | + | + | + |
| Hernia | − | + | + | − | − | + | + | + | − | − |
| Hip dislocation | − | + | + | − | − | + | + | − | + | + |
| Joint laxity | − | + | + | + | + | + | + | − | + | − |
| Corneal clouding | − | − | − | + | − | − | − | − | − | − |
| Pulmonary emphysema | − | + | − | − | − | − | − | − | − | − |
| Cardiac abnormalities | − | + | − | − | − | − | − | − | − | − |
| Urinary tract abnormalities | − | − | + | − | + | + | + | + | + | − |
| Occipital horns | − | − | − | − | + | − | − | − | − | − |
| Abnormalities of copper metabolism | ? | +/− | +/− | +/− | + | ? | ? | ? | ? | + |
- AD, autosomal dominant; XR, X-linked recessive; M, male; F, female; +, present; −, absent; ?, unknown.
Mutation Analysis of LOX, FBLN4, and FBLN5
Genetic studies were carried out according to institutional guidelines and after informed consent obtained in accordance with the Helsinki agreement. The study was approved by the Ethics Committee on Research, FCM/Unicamp, and the three patients, parents and control individuals participated in this study after giving informed consent. Total genomic DNA was extracted using standard techniques [Woodhead et al., 1986].
All seven exons including the exon–intron junctions of the LOX gene were PCR amplified and submitted to direct DNA sequencing. Primers and the PCR conditions are shown on Table II. The same strategy was used for the 10 exons of FBLN4 gene with the primers described by Hucthagowder et al. 2006 except for exons 1 and 2 that were grouped by the primer sequences (5′–3′) forward (TACAGGGAGTTGAGGTGCCG) and reverse (GTCCCTTGGAAAGCAGCTATC), and for the 11 exons of FBLN5 gene (Table III). PCR was performed in a total volume of 40 µl, containing 200 ng of genomic DNA, 20 pmol of each primers, 200 µM of each dNTP, 1.5 mM MgCl2, and 2.5 U of Taq DNA polymerase. The conditions for the reactions were 94°C for 5 min, 30 cycles at 94°C for 1 min, annealing temperature for 1 min, 72°C for 1 min, and a final extension at 72°C for 5 min. Direct sequencing was performed on an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit (ABI PRISM/PE Biosystems, Foster City, CA) and the products resolved on ABI PRISM™ 377 (Perkin Elmer, Boston, MA), according to the manufactures recommendations. The PCR primers were also used as sequencing primers.
| Exons | Sequence forward 5′–3′ | Sequence reverse 5′–3′ | AT (°C) | Product of PCR (bp) |
|---|---|---|---|---|
| 1a | GTCTCAGGATGTGTGTTCCG | CACGTGAGGGAAGGAGAAATC | 54 | 441 |
| 1b | GTGGAGACTGAGATACCCG | CAGGCTCAGCAAGCTGAAC | 57 | 315 |
| 1c | CCAGCAGATCCAATGGGAG | GGTTACTGAGCGCAGGAAC | 56 | 341 |
| 1d | GCTGGCTACTCGACATCTAG | CACGTCGAGAAGCCACATAG | 55 | 321 |
| 2 | CCGGGCGTCCCCAGTTCT | CCAGCTCTTGTCCCACTTCCTAAC | 61 | 380 |
| 3 | GAAAGGAGGATTGTCACTAC | CCTTCAGGTAAGAAATAAGAC | 55 | 337 |
| 4 | CTCTGTATGTAACTGACCACC | CTCTCTGAGGCTTGAGGTTC | 62 | 246 |
| 5 | TCATAGTCTGACCACATACC | CATTTGAATCCAGAGAAGAG | 58 | 220 |
| 6 | GTCTCCAGAGTTTAACCAC | GTAGCAGAAACAGATACATTC | 55 | 339 |
| 7a | GAGGGAAACTGTTGCATAAAG | CCAGTTATGTGCTTTGTTATTG | 55 | 280 |
| 7b | AAACTCCCAATGGATAAATCAG | TCTCAGCACCAGATGTGTCC | 57 | 483 |
- AT, annealing temperature; bp, base pair. Primers for exon 2 were described by Loeys et al. 2002.
| Exons | Sequence forward 5′–3′ | Sequence reverse 5′–3′ | AT(°C) | Product of PCR (bp) |
|---|---|---|---|---|
| 1 | AACCAGGTGCTTGCGCTGAG | TATCCTGACACCGCCTGAATC | 57 | 332 |
| 2 | ATATCACCGTTACACGTCAC | ACTGTAAAGCCACTCCCAC | 57 | 263 |
| 3 | GTGTGAAATGACCTTGCCTG | CACTGCAACTGGGCTGAATG | 58 | 152 |
| 4 | CCACTAATGCTCGCCCTTTC | GGTGAGCATGCCAGATACAG | 57 | 360 |
| 5 | AGGGCTCTAGATGGATGTG | CCTGGCAACCGTTAACTTC | 57 | 229 |
| 6 | GCAATCATGCCATGCTAGTGG | CCAGGTGCTGAAGAAGGCTG | 59 | 259 |
| 7 | GTGGTGGTAGATTTCGTTGC | GAAACACCTATGAAGGAAGC | 55 | 302 |
| 8 | GTAGATGGCCACTAAGCTCC | CAAAGCTGCACATGATTCCC | 55 | 297 |
| 9 | GTAGTAAGATCCAGCCTACC | GCACATGACGTAGGTAGTAG | 57 | 345 |
| 10 | CACCTGGAATGGATTTCTTG | GCATACAGCACGTTCCTAG | 55 | 321 |
| 11a | GTTTGACTGACGGAGGCAAG | GGGTGACAGGTCTGCAATAG | 60 | 432 |
| 11b | ACAGACGTTAGGCATTTCC | TGAACAAGACAGAATTAAACCG | 57 | 522 |
| 11c | AAACCACAGCAGGATCGAAG | TCTAAGCCAGCGCACAATAAAC | 56 | 419 |
- AT, annealing temperature; bp, base pair.
The restriction analysis was performed using the enzyme Taq I for the identification of a Ile315Ile polymorphism in FBLN5 and the enzyme Hinc II for the identification of a Ile259Val polymorphism in FBLN4.
RESULTS
Analysis and sequencing of FLNB5 did not show any abnormalities that were thought to modify the protein structure. However Patients 1 and 2 were both homozygous for a c. 945 T > C base substitution (Ile315Ile) in exon 9 of FBLN5, a previously published SNP (rs2430347). This SNP is a silent polymorphism and was detected in 41 of 50 individuals in our healthy control sample.
Screening of FBLN4 revealed a homozygous missense alteration (c. 776A > G; Ile259Val) in Patients 2 and 3 while Patient 1 was heterozygous for this same substitution; this is also a previously described SNP (rs601314). Since this alteration could be detected by restriction analysis using digestion with Hinc II, 50 unrelated healthy volunteers were studied and 49 individuals were identified with the G/G genotype. Therefore, this was considered another polymorphism. Finally, we also identified three intronic mutations.
The results of FBLN4 and FBLN5 gene analysis for the three families are summarized on Table IV. No alteration was found in the LOX gene in ARCL type II patients and their relatives.
| Genes | Finding | Change | SNPs | Family 1 | Family 2 | Family 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Mother | Patient 2 | Mother | Father | Patient 3 | Mother | ||||
| FBLN5 | Ile315Ile | T → C | rs2430347 | CC | CC | CC | CC | CT | TT | CT |
| FBLN4 | Ile259Val | A → G | rs601314 | AG | AG | GG | GG | GG | GG | GG |
| FBLN4 | His92His | C → T | rs633800 | CT | CC | TT | TT | TT | CC | CC |
| FBLN4 | IVS5 + 23 | G → C | rs630394 | GC | — | CC | — | — | CC | — |
| FBLN4 | IVS7 + 55 | T → C | rs501630 | TC | — | CC | — | — | TT | — |
| FBLN4 | IVS10 − 33 | C → T | rs594689 | CT | — | TT | — | — | CC | — |
- —, not tested.
DISCUSSION
Cutis laxa is the name given to a group of congenital conditions with important structural defects in elastic fibers. Biochemical studies show that different types of cutis laxa may also present with abnormalities in serum and urinary copper, ceruloplasmin and lysyl oxidase [Agha et al., 1978; Jung et al., 1996; Khakoo et al., 1997]; although not present in all individuals and not documented in many reports. These findings support the idea that low serum copper level produces a low elastase inhibitor substance with increased destruction of elastic fibers [Verma and Agrawal, 2003]. In Patient 3, both copper and ceruloplasmin were below the normality. This might reflect the abnormal elastic fibers detected histologically as previously suggested.
The study of Loeys et al. 2002 showed that a missense mutation Ser227Pro in FBLN5 causes ARCL type I with abnormal formation of elastic fibers in homozygotes in a single family. Their findings were corroborated by animal studies that confirm the importance of FBLN5 gene in the formation of those fibers [Nakamura et al., 2002; Yanagisawa et al., 2002]. Markova et al. 2003 reported an in tandem duplication causing a larger transcript due to an internal duplication of 483 nucleotides. This abnormality resulted in the synthesis and secretion of a mutant fibulin 5 protein which was associated with an autosomal dominant cutis laxa phenotype. Recently, Hucthagowder et al. 2006 identified a Glu57Lis missense mutation in FBLN4 in a patient with ARCL. Fibulins 4 and 5 clearly have an important role in elastogenesis.
In the present study, sequencing of all codifying region of FBLN5 and FBLN4 genes was carried out in three patients with ARCL type II. No mutations were observed. A polymorphism in exon nine (Ile315Ile) was demonstrated but this was frequent in control population. Heterozygosity for this same alteration was described by Wopereis et al. 2005 in three patients with type I ARCL and was also not thought to be associated with the abnormal phenotype. The sequencing analysis of the FBLN4 gene also identified the alteration Ile259Val (c. 776A > G). Although it involves a protein sequence change, we suggest that this SNP is unlikely to be pathological. The SNP frequency in our 50 controls was similar to that seen in Asian and European populations and shows only minor differences with African populations [International Hapmap Consortium, 2005]. The other SNP observed in the FBLN4 gene (His92His) does not change the protein structure. The same is true for the intronic mutations IVS5 + 23 G > C, IVS7 + 55 T > C, IVS10-33 C > T.
Therefore, all SNPs found in the present study are unlikely to be associated with disease and present allelic frequencies similar to other population studies. Finally, lysyl oxidase deficiency was described by Khakoo et al. 1997 in two individuals with a clinical picture of type II ARCL, although no similar investigation has been performed in other patients. This is the first time that LOX has been studied in this condition, and no mutation or SNP was found in our subjects.
More recently, Kornak et al. 2008 described mutations in ATP6V0A2 in 12 among 15 consanguineous families with ARCL type II/wrinkly skin syndrome. This gene encodes the a2 subunit of the V-type H+ ATPase which may result in defective glycosylation of protein(s) involved in elastic fiber formation, although the exact biochemical defect is yet to be elucidated. Ten different mutations were reported, which included five mutations leading to a premature stop in the N terminus while the remaining caused truncations in transmembrane segments. Thus, a complete loss of function of this enzyme is proposed by these authors. This is a potential candidate gene in the families presented in the paper.
Acknowledgements
This research was supported with grants from the State of São Paulo Research Foundation (FAPESP). Mr. Scherrer received a Master Fellowship from the National Council for Scientific and Technological Development (CNPq), Brazil.




