A girl with neurofibromatosis type 1, atypical autism and mosaic ring chromosome 17†
How to cite this article: Havlovicova M, Novotna D, Kocarek E, Novotna K, Bendova S, Petrak B, Hrdlicka M, Sedlacek Z. 2007. A girl with neurofibromatosis type 1, atypical autism and mosaic ring chromosome 17. Am J Med Genet Part A 143A:76–81.
Abstract
We describe a girl with neurofibromatosis type 1 (NF1), mild dysmorphic features, growth and mental retardation, autism, and mosaicism of ring chromosome 17 and chromosome 17 monosomy. The extent of genetic material deleted from the ring chromosome was determined using a combination of classical cytogenetics, fluorescence in situ hybridization (FISH) and multiplex ligation-dependent probe amplification (MLPA) to be 0.6–2.5 Mb on 17p, and up to about 10 Mb on 17q. Based on our observations and on a review of the literature we argue that in addition to a universal “ring syndrome” which is based on ring instability and is less specific for the chromosome involved, various ring chromosomes underlie their own characteristic phenotypes. We propose that the symptoms leading to the diagnosis of NF1 in our patient could be attributed to mosaic hemizygosity for the NF1 gene in some of her somatic cells. A similar mechanism or a direct involvement of respective disease genes in the aberration could possibly influence also the development of autism and other symptoms. We raise a question if the loss of one copy of chromosome 17 from a substantial fraction of somatic cells can have specific consequences also for future risks of the patient, for example, due to the mosaic hemizygosity for the BRCA1 and TP53 genes. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Chromosomal aberrations are found in about 10–15% patients with dysmorphic features, up to 20% patients with mild or moderate mental retardation, and up to 5% patients with autism [Raynham et al., 1996; Nussbaum et al., 2001; Castermans et al., 2004]. Among the aberrations, ring chromosomes are very rare. They arise due to breaks on both arms of a chromosome, and circular re-joining of the free ends. Because of problems with proper segregation during mitosis, these chromosomes are usually unstable, and mosaics of cell lines with loss of the rings, double rings, isochromosomes, or other marker chromosomes derived from the ring are often present in carriers of this aberration [Vogel and Motulsky, 1997].
There is a debate in the literature about the mechanism of how ring chromosomes influence the phenotype. One possibility is that virtually all abnormal clinical findings in the carriers are a consequence of the ring instability. The existence of a “ring syndrome” was proposed where most of the phenotype is not specific for the chromosome involved, and where the severity of the phenotype rather depends on the degree of ring instability, commonly involving growth failure due to increased mortality of the aneuploid cells [Kosztolanyi, 1987]. The other view is that the phenotype of the ring chromosome carriers is much more dependent on the genetic content of the chromosome involved, and that no universal “ring syndrome” exists. Various syndromes are described as separate clinical entities differing substantially in the degree of mental retardation, presence and severity of seizures, specific dysmorphic features, and presence of different organ defects (e.g., ring chromosome 13 syndrome, ring chromosome 20 syndrome, etc.) [Schinzel, 2001].
In this report, we present a girl whose relatively mild phenotype (neurofibromatosis type 1 (NF1), mild dysmorphic features, mental retardation, and autism) prompted us to perform a cytogenetic examination. We found mosaicism of ring chromosome 17 and chromosome 17 monosomy. As deletions in the NF1 gene were excluded in this patient, we suggest that the symptoms leading to the diagnosis of NF1 could be attributed to mosaic hemizygosity for the NF1 gene. We also raise a question if the loss of chromosome 17 from a substantial fraction of somatic cells of the patient can have a specific influence on the other abnormal clinical findings, and on her future specific risks.
MATERIALS AND METHODS
Clinical Report
The propositus was born by a spontaneous delivery at 41 weeks of gestation as a first child to a 21-year-old woman and a 22-year-old man. The birth weight of the girl was 2,950 g (25–50th centile), and length was 48 cm (25th centile). The newborn had slightly marked mongoloid slant of palpebral fissures, cafe-au-lait spots, and depigmentation on the belly. Early postnatal development was uncomplicated until 4 months of age when the girl started to exhibit feeding difficulties and failure to thrive. Her psychomotor development was delayed from the beginning (sitting with support from the age of 1 year, first steps from the age of 18 months). Speech development was slightly delayed (first words after 1 year of age, simple sentences after 3 years of age, severe dyslalia). At the age of 4 years she developed epilepsy. A paternal uncle of the girl's mother committed suicide, the paternal grandfather of the girl's mother suffered from alcohol abuse, and a maternal cousin of the girl's mother had epilepsy. There was no family history of NF1 or mental retardation.
The propositus (Fig. 1) was next examined at the age of 6.5 years. Her height was 104 cm (<3rd centile), weight was 16.5 kg (<3rd centile), and head circumference was 46.5 cm (<3rd centile). She had mild dysmorphic features with brachycephaly, bitemporal narrowing, wide nasal bridge, narrow palpebral fissures, epicanthal folds, thin upper lip, widely spaced teeth, down-turned mouth corners, and low-set dysplastic ears. She presented with mild scoliosis, and her gait was wide-based. Multiple café-au-lait spots on the skin increased with age, and mild axillary and inguinal freckling was present. These findings led to the diagnosis of NF1. No cutaneous neurofibromas or plexiform neurofibromas were detected. In addition, the girl had multiple depigmented macules (leukoderma), numerous naevi depigmentosi, and a planary naevus spilus in the right lumbar region. She also had fifth finger clinodactyly and bilateral halluces valgi.
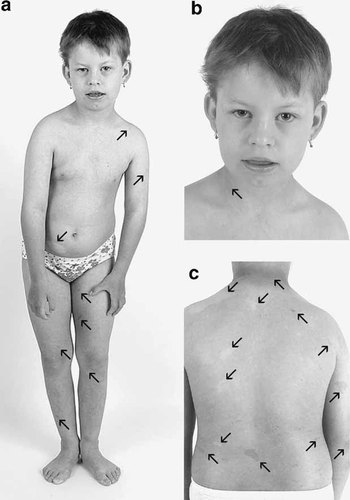
The propositus. a: Girl at the age of 9.5 years displaying mild dysmorphic features, short stature, scoliosis and skin pigmentary changes; (b) detailed frontal view of her face, demonstrating bitemporal narrowing, wide nasal bridge, narrow palpebral fissures, epicanthal folds, thin upper lip, down-turned mouth corners, and low-set dysplastic ears; (c) a detail of skin pigmentary changes on the back of the patient. The most pronounced café-au-lait spots and hypopigmented macules are marked by arrows pointing upwards and downwards, respectively.
We followed her for 4 years. The mild facial dysmorphism did not change during this period, but the growth deficiency became more pronounced. The seizures worsened significantly and were classified as atypical absence epilepsy and/or astatic epilepsy, becoming intractable with poor response to anticonvulsants. Finally, after combined antiepileptic therapy she was totally free of seizures from 9.5 years of age. On neurological examination at the age of 10 years she exhibited mental retardation, autistic features, and cognitive and language delay. She was awkward with a slow-paced gait. Other parameters were normal. Ophthalmologic examination revealed neither Lisch nodules (iris hamartomas), nor yellow flecks in the retina. Electroencephalography identified a right-sided focus of epileptiform activity in the parieto-temporal area. Magnetic resonance imaging of the brain showed no pathology. Optic glioma and high-signal intensity foci on T2-weighted images were absent.
The development of the behavioral phenotype of the girl was especially remarkable. The first psychiatric examination was performed at the age of 6.5 years. Hyperactivity and inattentiveness were the dominating symptoms. The clinical picture was not typically autistic at that time. The patient was seen by a psychiatrist at the age of 10.5 years. The reason for the admission were pronounced changes in her behavior (inappropriate smelling of things, increased aggressiveness, negativism, decreased perception of pain leading to occasional non-intentional self-injury, and an obsessive interest in observing animals and mimicking their sounds). The CARS (Childhood Autism Rating Scale) score combined with ADI-R (Autism Diagnostic Interview—Revised) results confirmed the diagnosis of atypical autism. Mental retardation was classified as moderate (IQ 45).
Cytogenetic Analysis, Fluorescence In Situ Hybridization (FISH) and Multiplex Ligation-Dependent Probe Amplification (MLPA)
Classical cytogenetic examination of G-banded chromosomes from peripheral blood lymphocytes was performed using standard protocols. FISH analysis employed a chromosome 17 centromeric probe (D17Z1), subtelomeric probes D17S2199 and D17S2200, and probes detecting the Miller-Dieker syndrome (MDLS) region (LIS1), the TP53 gene (p53), and the Smith-Magenis syndrome region (FL1), according to the instructions of the manufacturers (Cytocell or Vysis). MLPA assays [Schouten et al., 2002] were performed to analyze the presence of subtelomeric regions on the ring chromosome using kits P019/P020, and to detect deletions in the NF1 gene using kits P081/082, according to the instructions of the manufacturer (MRC-Holland, Amsterdam, The Netherlands).
RESULTS
Cytogenetic examination of 100 mitoses showed a mosaic karyotype 46,XX,r(17)(p13;q25)[83]/45,XX,-17[12]/47,XX,r(17), + r(17)[1]/46,XX,-17, + mar1[2]/46,XX,-17, + mar2[1]/46,XX,-17, + mar3[1]. The breakpoints on the ring chromosome were located in the terminal light G-bands 17p13 and 17q25 (Fig. 2), which both correspond to about 10 Mb of DNA. The marker chromosomes differed in size, and were most likely derived from chromosome 17 (the largest were probably isochromosomes of 17q). The parents did not give consent to perform skin biopsy to asses the degree of mosaicism in another tissue of the patient. Both parental karyotypes were normal.
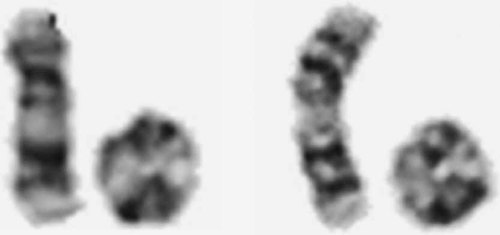
Classical cytogenetic analysis. Two representative pairs of the normal and ring chromosome 17 from G-banded metaphases.
FISH analysis showed that regions homologous to the subtelometric probes used for both arms (D17S2199 and D17S2200, located about 60 and 90 kb from the tip of 17p and 17q, respectively [Knight et al., 2000]) were deleted from the ring, while the MDLS gene, which maps approximately 2.5 Mb from the 17p telomere, was present (Fig. 3). Hybridization signals of all other chromosome 17 probes tested were also present on the ring chromosome. The results of the FISH analyses of the ring chromosome 17 can be described as 46,XX,r(17).ish r(17)(D17S2199-,LIS1 + ,TP53 + ,FL1 + ,D17Z1 + ,D17S2200-).
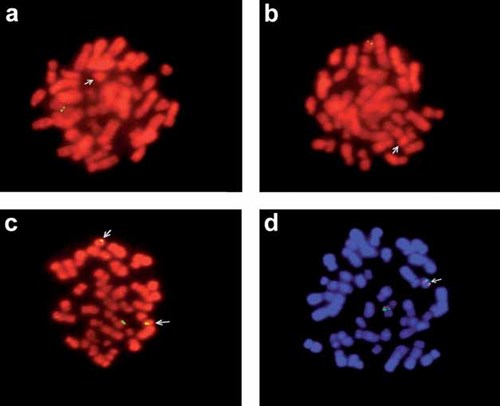
FISH analysis. a, b: Two metaphases hybridized with the 17p and 17q subtelomeric probes, respectively, showing only one signal from the normal chromosome 17, and no signals from the ring chromosomes (arrows); (c) a metaphase with one normal and two ring 17 chromosomes (arrows) hybridized with the TP53 probe, showing signals on all three chromosomes; (d) a metaphase hybridized with the Smith-Magenis region probe (green) and with the Miller-Dieker region probe (red), showing signals of both probes both on the normal chromosome 17 and on the ring (arrow).
Genomic DNA MLPA analysis of subtelomeric regions showed the absence of one copy of both chromosome 17 test genes (GEMIN4 and SECTM1). Compared to a normal control, the peak area was reduced to 53% and 47% for the signals from GEMIN4 and SECTM1, respectively (Fig. 4). This analysis excluded the breakpoints on the ring chromosome from the most distal 640 and 700 kb of 17p and 17q, respectively. The signals from all other subtelomeric regions did not differ from the control by more than 35% as recommended as an indication of a copy number change in MLPA [Schouten et al., 2002]. Similarly, the MLPA analysis of gains and losses in the NF1 gene was negative indicating that this gene was not affected by any gross deletions or deletions of single exons in the patient (data not shown).

MLPA analysis of subtelomeric regions. A part of the results obtained from a control DNA (a) and from DNA of the patient (b). The peaks correspond to individual subtelomeres. Signals from both subtelomeres of chromosome 17 are significantly reduced in the patient.
DISCUSSION
We describe a girl with mosaic non-supernumerary ring chromosome 17. The extent of the missing genetic material, as delineated by a combination of cytogenetics, FISH and MLPA, was 0.6–2.5 Mb on 17p, and up to about 10 Mb on 17q. Structural abnormalities of chromosome 17, and ring chromosomes of 17 in particular, are rare. Since the first description of a patient with ring chromosome 17 [Petit and Koulischer, 1971] only a few additional cases have been reported in the literature (reviewed in Shashi et al. 2003 and Endo et al. 1999). It is becoming evident that while patients with a deletion involving the Miller-Dieker region of 17p13.3 have lissencephaly, multiple dysmorphic features, severe mental retardation, and shortened life expectancy, patients who retain this region have a milder phenotype involving mild mental retardation, seizures, growth retardation, mild dysmorphism, and skin pigmentation changes [Shashi et al., 2003]. Our patient did not have a deletion of the Miller-Dieker region, and this finding was in accordance with her relatively mild clinical presentation.
Spectrum and severity of symptoms in patients with ring chromosomes can be determined by two factors. First, the aberration itself can influence gene expression in multiple ways. Genes distal to the breakpoints are lost, and patients can suffer from haploinsufficiency or from unmasking of recessive mutations on the remaining alleles. Genes spanning the deletion/fusion breakpoints can be interrupted or fused, resulting in possible gain-of-function effects. Finally, genes located in the vicinity of the breakpoints can be separated from their regulatory regions. The impact of chromosome circularization on the phenotype may thus vary depending on the size and content of the deleted segments, and on the location and nature of the breakpoints. Second, due to problems with chromatid separation, ring chromosomes are mitotically unstable, and give rise to a spectrum of secondary chromosomal defects including monosomy, double rings, and marker chromosomes [Vogel and Motulsky, 1997]. Various cell lines coexist within the patient in a mosaic state, and the phenotype can depend on the composition and frequency of mosaicism, and its distribution in different tissues [Nishiwaki et al., 2005]. Because of the instability of the ring chromosomes it was proposed that carriers, irrespective of the chromosome involved, suffer from a universal “ring syndrome” [Kosztolanyi, 1987]. The main symptom is growth retardation due to increased mortality of the aneuploid cells, accompanied by mild mental retardation, dysmorphism, and pigmentation changes.
The presence of the above symptoms in carriers of different ring chromosomes often mistakenly lead to the diagnosis of NF1. Our patient meets the NF1 criteria [Gutmann et al., 1997] with café-au-lait spots and axillary and inguinal freckling. The diagnosis of NF1 on the background of mosaicism for chromosome 17 monosomy raises the question if the skin pigmentation anomalies arise as an unspecific manifestation of the “ring syndrome,” or if this phenotype is specifically related to the loss of one copy of the NF1 gene (which maps to 17q11) from the population of monosomic cells. It is conceivable that the 12% mosaicism for chromosome 17 monosomy identified in blood of the patient can have consequences similar to those described in patients with NF2 who have 6% or even lower mosaicism for monosomy of chromosome 22 (and of the NF2 gene in 22q12) [Tsilchorozidou et al., 2004]. This conclusion is further supported by excluding a coincidental independent rearrangement of the NF1 gene in our patient by MLPA analysis.
In addition to several patients with ring chromosome 17 and NF1 (reviewed in Shashi et al. 2003), a patient with this aberration and carcinoma of breast and ovary has been described [Wiktor et al., 1993]. Similarly, several cases of ring chromosome 22 were associated with NF2 and increased cancer susceptibility [Luciani et al., 2003; Tsilchorozidou et al., 2004]. Cancer was also associated with ring chromosomes 11 and 13 [Tommerup and Lothe, 1992]. Similar to the latter authors, we propose that these findings may not be chance coincidences, and that the phenotypes are caused by loss of one copy of the respective disease gene (BRCA1, NF2, SNF5 or CHEK2, WT1, and RB1) in monosomic cells arising due to ring instability. Resolving the question of specific symptoms associated with specific ring chromosomes is very important from the clinical point of view. Our patient could, for example, be at increased risk of cancer due to her mosaicism for cells with loss of one allele of TP53 and BRCA1, similar to patients with germline mutations in these tumor suppressor genes.
Our patient was also diagnosed with atypical autism and mental retardation. Although these conditions may belong to non-specific symptoms associated with many chromosomal aberrations, it is interesting that several genome screens pointed to various portions of chromosome 17q or 17p as to potential regions harboring autism susceptibility genes [Risch et al., 1999; IMGSAC (International Molecular Genetic Study of Autism Consortium), 2001; Yonan et al., 2003], and that breakpoints in 17p13 have been observed in Asperger syndrome [Tentler et al., 2003]. It is tempting to speculate about a causal link here as well.
Deletions in regions with imprinted genes can also have consequences potentially even more serious than those associated with loss of a biparentally expressed gene. It may be of interest in this context that the most terminal part of 17q, which may be deleted in our patient, was suggested to contain maternally imprinted gene(s) [Rio et al., 2001]. Finally, as it becomes more and more recognized for broadening spectra of genetic disorders, the phenotypic effects of any genetic defect are likely to be influenced by the genotype of the patient at other loci (genetic background), and by environmental factors. These factors can underlie a part of the inter-individual phenotypic variability observed among the ring chromosome carriers.
In conclusion, the clinical and laboratory findings in our patient suggest that although some phenotypic features observed could be a non-specific manifestation of the “ring syndrome,” her diagnosis of NF1 may be specifically related to her mosaic chromosome 17 monosomy and loss of one of the copies of the NF1 gene from a significant proportion of somatic cells. This mechanism or a direct involvement of respective disease genes in the aberration may play a role also in the development of other symptoms including autism.




