Monoallelic BUB1B mutations and defective mitotic-spindle checkpoint in seven families with premature chromatid separation (PCS) syndrome
Abstract
Cancer-prone syndrome of premature chromatid separation (PCS syndrome) with mosaic variegated aneuploidy (MVA) is a rare autosomal recessive disorder characterized by growth retardation, microcephaly, childhood cancer, premature chromatid separation of all chromosomes, and mosaicism for various trisomies and monosomies. Biallelic BUB1B mutations were recently reported in five of eight families with MVA syndrome (probably identical to the PCS syndrome). We here describe molecular analysis of BUB1B (encoding BubR1) in seven Japanese families with the PCS syndrome. Monoallelic BUB1B mutations were found in all seven families studied: a single-base deletion (1833delT) in four families; and a splice site mutation, a nonsense mutation, and a missense mutation in one family each. Transcripts derived from the patients with the 1833delT mutation and the splice site mutation were significantly reduced, probably due to nonsense-mediated mRNA decay. No mutation was found in the second alleles in the seven families studied, but RT-PCR of BUB1B and Western blot analysis of BubR1 indicated a modest decrease of their transcripts. BubR1 in the cells from two patients showed both reduced protein expression and diminished kinetochore localization. Their expression level of p55cdc, a specific activator of anaphase-promoting complex, was normal but its kinetochore association was abolished. Microcell-mediated transfer of chromosome 15 (containing BUB1B) into the cells restored normal BubR1 levels, kinetochore localization of p55cdc, and the normal responses to colcemid treatment. These findings indicate the involvement of BubR1 in p55cdc-mediated mitotic checkpoint signaling, and suggest that >50% decrease in expression (or activity) of BubR1 is involved in the PCS syndrome. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Cancer-prone syndrome of premature chromatid separation (PCS) with mosaic variegated aneuploidy (called PCS syndrome hereafter) is a rare autosomal recessive disorder, characterized by premature separation of sister-chromatids of all chromosomes in more than 50% of mitotic lymphocytes; and a variety of mosaic aneuploidies, especially trisomies, double trisomies, and monosomies, a finding called mosaic variegated aneuploidy (MVA) [Kajii et al., 1998, 2001]. Infants with the PCS syndrome show pre- and postnatal growth retardation, profound developmental delay, severe microcephaly, hypoplasia of the brain with Dandy–Walker complex or other malformations of the posterior fossa, cataracts, uncontrollable clonic seizures, and a high risk of malignancy including Wilms tumor, rhabdomyosarcoma, and leukaemia. Some two dozen probable or definite cases have been reported worldwide [Kajii et al., 2001; Chen et al., 2004]. The parents of the patients with the PCS syndrome are phenotypically normal, have 2%–47% lymphocytes in PCS, but are without MVA.
We reported that cultured skin fibroblasts from two unrelated infants with the PCS syndrome did not arrest at metaphase after treatment with colcemid, and entered G1 and then S phase without sister-chromatid segregation or cytokinesis [Matsuura et al., 2000]. These results indicated that the infants' cells were insensitive to the colcemid-induced mitotic-spindle checkpoint. Mitotic-spindle checkpoint is a surveillance mechanism that delays the metaphase to anaphase transition until all chromosomes have established bipolar attachment to the microtubules, thus ensuring accurate sister chromatid segregation during each cell division [Taylor et al., 2004].
A number of yeast components involved in mitotic spindle checkpoint have been isolated. Human counterparts (Mps1, Bub1, Bub3, BubR1, Mad1, Mad2, and CENP-E) of the yeast components have also been characterized. Cellular and biochemical studies demonstrated that these products are part of a molecular signaling cascade that, by inactivating an anaphase-promoting complex (APC) and its specific activator p55cdc, blocks premature sister-chromatid separation. Once all the chromosomes attach to the spindle and align at the metaphase plate, checkpoint activity ceases and the APC becomes active, inducing sister-chromatid separation by securin protein proteolysis and exit from mitosis by cyclin B proteolysis [Clute and Pines, 1999; Cleveland et al., 2003]. Many of the checkpoint proteins and p55cdc are present in the cytoplasm of interphase cells and move to the kinetochore during an early stage of mitosis where they are postulated to participate in generating the wait anaphase signal [Kallio et al., 2002; Musacchio and Hardwick, 2002].
Hanks et al. [2004] recently analyzed ten patients from eight families with what they called MVA syndrome (probably identical to the PCS syndrome), and found biallelic mutations of the BUB1B gene in six patients from five families. In each of these five families, there was one mutation that resulted in premature protein truncation or an absent transcript, and one missense mutation. In all cases, the mutations were inherited from different parents.
We here describe identification of BUB1B mutations, and functional evaluation of BubR1, a protein it encodes, and mitotic checkpoint function in seven Japanese families with the PCS syndrome.
MATERIALS AND METHODS
Subjects
Detailed clinical and cytogenetic features of five of the seven families have been described previously: family 1 by Kawame et al. [1999], families 2, 4, and 7 by Kajii et al. [2001], and family 6 by Kajii et al. [1998]. Families 3 and 5 have not been reported previously. Family 3 involved a 4-month-old girl with intrauterine growth retardation (IUGR), annular pancreas, and duodenal obstruction, but without microcephaly or Dandy–Walker complex. Family 5 had a girl who had IUGR, clonic seizures, microcephaly, Dandy–Walker complex, and bilateral cataracts; developed botryoid rhabdomyosarcoma of the bladder and bilateral Wilms tumors; and died at age 9 months. The seven families included eight patients: one patient each in families 1–6, and two patients (patients 7A and 7B) in family 7 (Table I). Of the eight patients, five had Wilms tumors, and two developed Wilms tumors and embryonic rhabdomyosarcomas.
| Patient | Age at death (years) | IUGR | Microcephaly | Dandy–Walker complex | Cataracts | Other anomalies | Wilms tumor | ERMSa | PB lymphocytes | |
|---|---|---|---|---|---|---|---|---|---|---|
| PCS (%) | MVA (%) | |||||||||
| 1 | 3 | + | + | + | + | Corneal opacities | + | 82 | 17 | |
| 2 | 0.4 | + | + | + | − | + | 48.5; 73.5 | 9 | ||
| 3 | 0.3 (alive) | + | − | − | − | Annular pancreas | 17 | 12 | ||
| 4 | 8 (alive) | + | + | + | + | + | 68.5; 83.2 | 25 | ||
| 5 | 0.8 | + | + | + | + | + | + | 66.5 | 10 | |
| 6 | 1.5 | + | + | + | + | Cleft palate | + | 67–86.5 | 16.4 | |
| 7A | 1.1 | + | + | + | ? | + | + | 36b | 18b | |
| 7B | 0.6 | + | + | + | ? | + | ?b | 32b | ||
- a Embryonic rhabdomyosarcoma
- b Chromosome slides were prepared in an outside laboratory and analyzed by us.
Cell Lines
Immortalized cell lines were established by transfecting primary cultured fibroblasts with SV40 virus, and then transfecting the cells that entered crisis with phTERT retrovirus vector [Carney et al., 2002]. HCT116 cell line (a colorectal cancer cell line with microsatellite instability) and HeLa cells (a human cervical cancer cell line) were used as normal controls. EB virus-transformed lymphoblastoid cell lines were established from peripheral blood lymphocytes. Mouse hybrid cell line GM11715 containing human chromosome 15 was obtained from the NIGMS Human Genetic Mutant Cell Repository, and microcell-mediated chromosome transfer was performed as described previously [Matsuura et al., 1997].
BUB1B Mutational Analysis
PCR primers were designed to amplify all coding exons of BUB1B, at least 50 bp of the intronic sequence that contained the 5′ and 3′ splice junctions, the promoter region 5 kb upstream of the translation initiation site, and the 3′-untranslated region. RT-PCR primers for BUB1B cDNA were synthesized to cover the open reading frame with eight overlapping segments (primers and conditions available on request). PCR products were directly sequenced using a DNA sequencer 4000L (LI-COR Biosciences, Lincoln, NE). The patients and their parents were analyzed in families 1–5. In family 6, the parents, grandparents, and sibs of the patient were studied but patient 6 was unavailable. Likewise, in family 7 the parents but not the patients were available for study.
Haplotype Analysis
Large-scale genomic sequence data, including BUB1B, was obtained from the National Center for Biotechnology Information (NCBI) website. We identified a dinucleotide repeat sequence 26020GT, 54 kb upstream of the BUB1B gene. The 26020GT sequence was PCR-amplified with primers, 5′-CCTGGAGAATGAGGAGATGC-3′ and 5′-CACTTGGTCCTTTCATCAGGA-3′. PCR products were analyzed with a DNA sequencer 4000L (LI-COR Biosciences), and 12 alleles were numbered in the order of the respective size. A microsatellite marker D15S994, situated 69 kb downstream of the gene, was obtained from the NCBI website and used in this study. The frequencies of these alleles in the Japanese general population were determined in 184 chromosomes of unrelated individuals. Single nucleotide polymorphism 1046A/G (encoding 349 Gln/Arg) at exon 8 of BUB1B was determined by sequencing the gene [Katagiri et al., 1999].
Western Blot Analysis
Cells were harvested and lyzed in 1× RIPA buffer. Protein samples were resolved through 8% SDS–PAGE, and transferred onto Hybond-P (GE Healthcare, Chalfont St. Giles, UK). The membranes were incubated with primary antibody in PBST, and with 1:30,000 secondary antibodies conjugated to Alexa Fluor 680 or IR-dye 800 (Molecular Probes, Eugene, OR). The membranes were analyzed using Odyssey Infrared Imaging System (LI-COR Biosciences). Band intensity was estimated using a densitometer. Primary antibodies used were: rabbit anti-MAD2 polyclonal antibodies (Covance, Princeton, NJ); mouse anti-Bub1 monoclonal antibodies (Chemicon International, Temecula, CA); mouse anti-Bub3 monoclonal antibodies (BD Biosciences, Franklin Lakes, NJ); rabbit anti-p55cdc polyclonal antibodies (Santa Cruz); mouse anti α-tubulin monoclonal antibodies (Sigma-Aldrich, St. Louis, MO). Rabbit anti-BubR1 polyclonal antibody was raised against human BubR1 (amino acids 1–478).
Cell Cycle Analysis
Immortalized fibroblast cells were grown on glass slides, and 0.05 µg/ml colcemid was added to the culture medium at the next day for 0, 6, 12, 18, 24, or 30 hr. Cells were fixed with 3:1 methanol: acetic acid, stained with Hoechst 33258, and analyzed under a fluorescence microscope. At least 300 cells were counted, including those with tightly condensed chromosomes and those with multiple small nuclei. Mitotic index was defined as the percentage of cells with tightly condensed chromosomes.
Immunofluorescence Analysis
Immortalized fibroblast cells were plated onto glass slides and used the next day for immunofluorescence staining. Cells were fixed with methanol at −30°C for 20 min, then with acetone at −30°C for 5 sec, and air-dried. Cells were treated with 10% bovine serum albumin in PBS(−) pH 7.2 for 20 min, and were incubated with primary antibodies for 1 hr at room temperature in a humidified chamber. After washing in PBS, cells were incubated with 1:1,000 secondary antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes), washed, and mounted with fluorescent mounting medium (DAKO, Carpinteria, CA) containing 0.5 mg/ml 4′, 6-diamidino-2-phenylindole (DAPI). Stained cells were visualized with either a 60× or 100× Zeiss objective lens mounted on a Zeiss Axioskop 2. Images were captured with a digital camera (Carl Zeiss, Munich, Germany) controlled by IPLab Spectrum (Scanalytics, Rockville, MD). The primary antibodies used were: rabbit anti-BubR1 antibodies; rabbit anti-Mad2 antibodies (Covance); mouse anti-Bub1 antibodies (Chemicon International); rabbit anti-p55cdc antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); goat anti-CENP-E polyclonal antibodies (Santa Cruz); and rabbit anti-CENP-F monoclonal antibodies (Novus Biologicals, Littleton, CO). Mouse anti-CENP-B monoclonal antibodies (2D8D8) and rabbit anti-CENP-C antibodies (CRa1) were gifts from Dr. H. Masumoto (Nagoya University, Japan).
RESULTS
BUB1B Mutations
Four heterozygous mutations were detected in the seven families (Table II, Fig. 1). Of these, 1833delT, a single-base deletion in exon 15 resulting in protein truncation, was observed in families 1, 5, 6, and 7: in patient 1 and her mother; patient 5; the brother, the father, and paternal grandmother in family 6; and in the mother in family 7. A splice site mutation, IVS10-5A > G, was detected in patient 2 and his father. RT-PCR analysis demonstrated that the splice site mutation resulted exclusively in an aberrant splicing in an alternative site 4-bp upstream of intron–exon junction site and leading to a premature termination codon. A single-base substitution, 670C > T, resulting in a nonsense mutation (R224X), was detected in patient 3 and her mother. Transcripts derived from the 1833delT and IVS10-5A > G mutations were drastically reduced (data not shown), possibly due to nonsense-mediated mRNA decay, and hence these alleles were effectively null. It is unknown whether in patient 3 the transcript from the 670C > T mutation is also subject to nonsense-mediated mRNA decay, because RNA from the patient was unavailable. Patient 4 and her mother had a single-base substitution in exon 2, 107G > A, which results in an amino acid substitution of R36Q. This missense mutation was located in the N-terminal, highly conserved domain (Fig. 2). The four mutations were all apparently monoallelic in the five patients analyzed. No mutation was found in the second allele in each of the five patients studied, including the full coding regions, intron–exon boundaries, the promoter region 5 kb upstream of the translation initiation site, and the 3′-untranslated region. Southern blot analysis showed no gross rearrangement in the second allele in the patients. cDNA sequencing indicated that the second allele expressed intact mRNA transcripts.
| Patient | Exon | Mutation | Type | Protein effect | Location in BubR1 protein | Inheritance |
|---|---|---|---|---|---|---|
| 1 | 15 | 1833delT | Frameshift | F611fsX625 | Maternal | |
| 2 | 11 | IVS10-5A > G | Splice site | Q468fsX480 | Paternal | |
| 3 | 6 | 670C > T | Nonsense | R224X | Maternal | |
| 4 | 2 | 107G > A | Missense | R36Q | N-terminus | Maternal |
| 5 | 15 | 1833delT | Frameshift | F611fsX625 | ? | |
| 6 | 15 | 1833delTa | Frameshift | F611fsX625 | Paternal | |
| 7A, B | 15 | 1833delTa | Frameshift | F611fsX625 | Maternal |
- a Deduced from the analysis of family members.

Pedigrees of families 1–7. The numbers and letters within the boxes represent allele type of 26020GT, 1046G/A, and D15S994. Mutations detected are shown under the boxes. Shadowed boxes represent BUB1B mutation-linked haplotypes, and hatched boxes indicate the haplotype linked to the second allele.
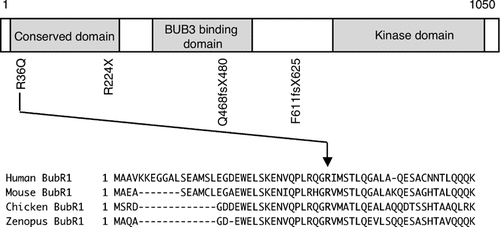
Scheme of the primary structure of BubR1 showing position of mutations identified in the patients. Under the scheme, amino acid sequences of BubR1 orthologs are aligned to show a maximum homology. The conserved 36th Arg residue is indicated.
We analyzed the seven families for microsatellite marker 26020GT situated 54 kb upstream of BUB1B; single-nucleotide polymorphism (SNP) 1046G/A (rs1801376) at exon 8 of BUB1B; and microsatellite marker D15S994, 69 kb downstream of the gene. Haplotype data of 26020GT-1046G/A-D15S994 were constructed in family 6, and those from the other nuclear families were inferred under assumption of existence of an ancestor chromosome. A 2A7 haplotype was linked to the 1833delT mutation in three of the four families analyzed, a finding that suggested a common origin of the mutation (Fig. 1). In the remaining family (family 7), it was 1A7 instead of 2A7. The haplotype in the second allele in which no mutation was detected was 6G3 in five of the seven families, and it was 5G3 or 7G3 in family 3 and 6G8 in family 5 (Fig. 1).
The frequency of SNP 1046G in 224 Japanese normal controls was 0.69, and that of 1046A was 0.31 [Katagiri et al., 1999]. Screening of 92 Japanese normal individuals indicated that the 26020GT maker to have12 types of alleles and the D15S994 marker to contain 11 types.
Expression of BubR1 Protein
Western blot analysis of BubR1 expression was performed on immortalized fibroblast cell lines from patients 1, 2, and 5, and in control HeLa and HCT116 cells, using rabbit anti-BubR1 polyclonal antibody raised against human BubR1. Mutation analysis of these cell lines predicted a combination of no protein expression due to nonsense-mediated mRNA decay and expression of intact 120-kD protein due to the second allele without detectable mutation. Consistent with this, BubR1 bands of normal size but reduced intensities (22%, 25%, and 29% of HeLa cells) were detected in these cell lines. Analysis of EB virus-transformed lymphoblastoid cell lines from family 2 (Fig. 3B) indicated band intensities to be in the order of the mother (6G3 haplotype, at 71% of a normal control), father (IVS10-5A > G, a protein truncating mutation, at 57%), and patient 2 (truncating mutation plus 6G3 haplotype, at 22%). Analysis of lymphoblastoid cells from eight members of family 6 (Fig. 3B, bottom) demonstrated I-4 and II-7, both carriers of the 6G3 haplotype, to show band intensities (38% and 31%) intermediate between the normal individuals (I-1, I-3, and III-1, at 112%, 158%, and 63%) and the carriers of the 1833delT mutation (I-2, II-2, and III-2, at 13%, 21%, and 29%). Taken together, these findings indicate that the expression of BubR1 was in the descending order of normal individuals (63%–158%), individuals with the 6G3 haplotype (31%–71%), those with a truncating mutation (21%–57%), and patients with a truncating mutation plus the 6G3 haplotype (22%–29%). Variations between individuals in each group were large and overlapping was seen among different groups, but the trend was noticeable.
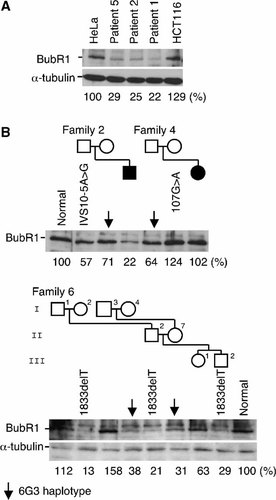
Western blot analysis of BubR1 protein expression in immortalized fibroblasts from patients 1, 2, and 5 (A), and in lymphoblastoid cells from families 2, 4, and 6 (B). Anti-α-tubulin antibody served for equal loading. Densitometric levels of the BubR1 bands are shown beneath the BubR1 and α-tubulin bands.
Family 4 was different in that the mother, with a missense mutation 107G > A (R36Q), showed an increased BubR1 band of 124% (Fig. 3B). Her daughter, patient 4, showed a normal band intensity of 104%, presumably because the effect of the mutation and that of 6G3 haplotype have canceled each other.
Mitotic Checkpoint Defects
Chromosome preparations from immortalized fibroblast cells from patient 1 showed 65% metaphases in PCS, a hallmark of a defective mitotic checkpoint, whereas those from a normal individual had only 0%–1% cells in PCS (Fig. 4A).
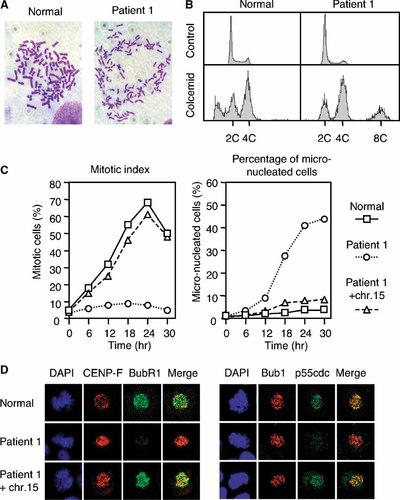
A: An immortalized fibroblast metaphase from a normal individual (left) and that in PCS from patient 1 (right). B: Flow-cytometric analysis of fibroblasts from patient 1 (right), without (top) and with (bottom) 72 hr colcemid treatment. TIG-3 cells (left) served as a normal control. C: Mitotic indices (left) and frequencies of micronucleated cells (right) after treatment with colcemid. HCT116 cells (□) showed an increase of mitotic index with a peak at 24 hr, whereas immortalized cells from patient 1 (○) showed no clear peak in mitotic index and increasing accumulation of micronucleated cells. Microcell-mediated transfer of a human chromosome 15 (including the BUB1B locus) into the cells from patient 1 (▵) restored the accumulation of mitotic cells and abolished the accumulation of micronucleated cells. D: Immunofluorescence analysis of metaphases from patient 1, and those containing transferred chromosome 15 (patient 1 + chr.15). HeLa cells served as a normal control. Cells in the left panel were stained with CENP-F (red) and BubR1 (green), and those in the right panel were stained with Bub1 (red) and p55cdc (green). Chromosomes were stained with DAPI (blue). The patient's cells showed diminished signals of both BubR1 and p55cdc on the kinetochores. Transfer of chromosome 15 into the patient's cells restored kinetochore association of both BubR1 and p55cdc.
Primary cultured skin fibroblasts from patients 1 and 2 were treated with 1 µg/ml colcemid for 72 hr, and flow-cytometric analysis was carried out [Ito et al., 1999]. Both control normal cells (TIG-3) and those from patient 1 without colcemid treatment (Fig. 4B, top) had a small peak at 4C. Control cells colcemid treated for 72 hr (Fig. 4B, bottom left) showed a large fraction below 2C, likely to represent apoptotic cells. Cells from patient 1 colcemid treated for 72 hr (Fig. 4B, bottom right) did not show any accumulation of the apoptotic cells, and instead, demonstrated a peak at 8C, an indication that these cells escaped from 4C. These results indicate that BubR1 is involved in colcemid-induced mitotic arrest, and suggest that it may play a role in apoptosis in cells that escaped mitotic arrest.
We examined the effect of colcemid treatment on the mitotic index of immortalized fibroblast cells from patients 1 and 2. Control HCT116 cells showed an increase of mitotic index with a peak at 24 hr, whereas the cells from the patients showed abnormal response with no clear peak in mitotic index (Fig. 4C, left) with an increasing accumulation of micronucleated cells (Fig. 4C, right). We tried to transfect these immortalized cells with a plasmid that encodes BUB1B cDNA, but were unsuccessful. Therefore, we introduced into these cell lines whole or a part of chromosome 15 (containing the BUB1B locus) through microcell-mediated transfer [Matsuura et al., 1997]. The transfected cells restored the accumulation of mitotic cells (Fig. 4C, left), and abolished the accumulation of micronucleated cells (Fig. 4C, right).
Mitotic Checkpoint Proteins
Immortalized fibroblast cells from patients 1 and 2 were analyzed for mitotic checkpoint proteins, with HeLa cells serving as a normal control. In the patients' cells, normal levels of Mad2, Bub1, and Bub3 proteins were detected with Western blot analysis (data not shown), and Mad2, Bub1, and CENP-B, -C, -E, and -F proteins localized to the kinetochores efficiently (Fig. 4D).
Western blot analysis of these patients' cells demonstrated reduced expression of BubR1 but normal levels of p55cdc protein (data not shown). Kinetochore signals of BubR1 were faint, and kinetochore p55cdc signals were markedly reduced (Fig. 4D, middle row). Transfer of chromosome 15 (containing BUB1B) into the patients' cells restored kinetochore association of both BubR1 and p55cdc (Fig. 4D, bottom). These results suggest that in these patients reduced expression of BubR1 caused abolished kinetochore localization of p55cdc and abnormal checkpoint signaling.
PCS Cells in Cultured Peripheral Blood Lymphocytes
Cultured peripheral blood lymphocytes in the individuals from the seven families were arrested with 0.1–0.4 µg/ml colcemid for 2 hr, treated with 0.075 M KCl at 32°C or 37°C for 20 min, and chromosome slides were prepared. PCS cells were 0%–1% in normal individuals, 1%–13% in the carriers of the 6G3 haplotype, 8.5%–47% in mutation carriers, and 48.5%–83.2% in the patients with a BUB1B mutation plus 6G3 haplotype. Patient 3, with a nonsense mutation, showed 17% PCS cells, an unusually low frequency (Table I). Cultures from each couple of parents were hypotonic treated in parallel and in the same manner. In each couple, the parent with a detectable mutation showed a higher frequency of cells in PCS than that without detectable mutation (Table III).
| Family | PCS cells in parents (%) | |
|---|---|---|
| Mutation positive | No detectable mutation | |
| 1 | 42.5 | 5 |
| 2 | 8.5 | 3.5 |
| 3 | 15 | 1a |
| 4 | 24.7 | 4.1 |
| 6 | 15.5 | 2.5 |
| 7 | 16 | 6.5 |
- a Hypotonic treated at 32°C for 20 min. It was 3% when treated at 37°C for 20 min.
DISCUSSION
We found monoallelic BUB1B mutations in all seven families with the PCS syndrome studied. They included a single-base deletion (1833delT) in families 1, 5, 6, and 7, a splice site mutation (IVS10-5A > G) in family 2, a nonsense mutation (670C > T) in family 3, and a missense mutation (107G > A) in family 4 (Table II, Fig. 1). Of these, the first three mutations were those that result in premature protein truncation, whereas 107G > A (R36Q) was a missense mutation. The missense mutation, however, is assumed to be functionally debilitating, being located in the N-terminal, and conserved domain (Fig. 2). The 1833delT deletion mutation was linked to a 2A7 haplotype of 26020GT-1046G/A-D15S994 in three (families 1, 5, and 6) of four families studied. We thus assume that the 1833delT mutation in these families was derived from a common, distant ancestor, although these families now live in widely separated places in Central and Southern Japan, and not known to be related with one another.
No second mutation was found in each of the five patients, in the allele opposite the one in which a mutation was detected, although all coding regions, intron–exon boundaries, the promoter region, and the 3′-untranslated region of the gene were sequenced. No alternately spliced BUB1B transcript from the second allele was detected with RT-PCR analysis. In each of the remaining two families (families 6 and 7) where the patients were unavailable, one parent carried a mutation, whereas the other parent did not. This was in apparent contradiction with the proposal by Hanks et al. [2004] that MVA syndrome is caused by biallelic BUB1B mutations. Hanks et al. [2004] observed biallelic mutations in MVA families that always consisted of paired protein truncating and missense mutations. The truncating mutations result in 50% reduction of BubR1 function. The missense mutations in the second allele most likely only disrupt the functions of the BubR1 protein in a subtle way. The paired protein truncating and missense mutations together induce >50% reduction of BubR1. They speculated that, referring to embryonic lethality in knockout mice, it was necessary to have at least one missense allele that allowed for some minimal production of BubR1 protein.
Several explanations are conceivable for the apparent contradiction. First, phenotypic and genetic differences may exist between the MVA syndrome described by Hanks et al. [2004] and the PCS syndrome we have dealt with. Both groups of patients had IUGR, microcephaly, eye anomalies and MVA. PCS was found in all PCS and most MVA patients. The PCS patients tended to have severe brain anomalies including Dandy–Walker complex, whereas only one MVA patient had cerebellar hypoplasia. Seven of eight PCS patients developed cancer: four patients had Wilms tumor, and two patients had Wilms tumor plus rhabdomyosarcoma. Of the 10 MVA patients, only 2 had rhabdomyosarcoma, and none had Wilms tumor. These differences, however, are likely to reflect collection and examination biases [Ikeuchi et al., 2004], and so we assume that the two syndromes are essentially identical.
The second possibility is that a monoallelic BUB1B mutation is enough to induce the PCS syndrome. This, however, is unlikely in view of the fact that the parents who carried the same monoallelic mutations as the PCS patients were phenotypically normal, except for the occurrence of <50% PCS cells in cultured lymphocytes.
The third possibility is that the second alleles in the PCS patients had undetectable mutations. This is rather unlikely because all coding regions, intron–exon boundaries, the promoter region, and the 3′-untranslated region were sequenced in seven families. Haplotype data suggests that there are at least three chromosomes without detectable mutations in these families. Yet, the possibility remains that these approaches are failing to detect several different mutations in BUB1B that coincidentally occur one to a family.
The fourth possibility is that there were no BUB1B mutations in the second alleles of the PCS patients studied, but their production of the BubR1 protein was compromised as a reflection of allelic variations in gene expression. The 26020GT-1046G/A-D15S994 haplotype of the second allele was 6G3 in five of the seven families studied (Fig. 1). The frequencies of these allelic markers in a normal Japanese population are, respectively, 7%, 69%, and 46.7%. Because linkage disequilibrium is expected to be operating between these relatively closely spaced markers, it would be safe to assume that the frequency of the 6G3 haplotype in the general population is 7%, the same level as that of the 26020GT marker. The probability of a haplotype with a frequency of 7% to occur in five of seven families is low (P < 0.00001). Western blot analysis of the PCS families indicated that the transcripts of the second allele are moderately reduced (Fig. 3). Yan et al. [2002], in a study of 13 genes, found 6 genes with considerable variations in expression. Individuals whose alleles were differentially expressed showed ratios of transcripts varying from 1.3:1.0 (FBN1) to 4.3:1.0 (p73). We thus prefer allelic variations of gene expression as the explanation for the apparent discrepancy between biallelic mutations in the MVA patients and monoallelic mutations in the PCS patients.
The frequencies of PCS cells in lymphocyte cultures from six couples of parents served to predict the parents with a mutation and those without a detectable mutation (Table III). In each couple, peripheral blood lymphocytes were cultured and processed in parallel and in the same manner. Our current protocol in lymphocyte cultures is addition of 0.1 µg/ml colcemid for 2 hr, and hypotonic treatment at 32°C for 20 min. Only when the frequency of PCS cells is unexpectedly low, we reexamine using 37°C, 20 min hypotonic treatment.
Western blot analysis of the BubR1 protein in the cells from these families indicated that its expression intensity is in the decreasing order of normal individuals; individuals with 6G3 haplotype; those with protein truncating mutations; and the patients with truncating mutations plus 6G3 haplotype. As mentioned earlier, the expression of the allele with protein truncating mutations in families 1 and 2 was effectively null. Heterozygous carriers of these mutations, therefore, would produce 50% reduced BubR1, and the patients with these mutations plus 6G3 phenotype would have more than 50% reduced BubR1, an assumption supported by our Western blot study.
We reported previously that cultured skin fibroblast cells from two PCS patients (patients 1 and 2 in this paper) were insensitive to colcemid-induced mitotic spindle checkpoint. Immortalized skin fibroblast cell lines from these patients showed essentially the same response to colcemid treatment as cultured skin fibroblasts, with fewer mitotic cells and no clear peak in mitotic index (Fig. 4B,C). These results support our previous suggestion that the PCS syndrome is a disorder of mitotic-spindle checkpoint [Matsuura et al., 2000]. Microcell-mediated transfer of whole or part of chromosome 15 containing the BUB1B locus into these immortalized cells restored their normal response to colcemid treatment, a finding to support BUB1B mutations as the cause of the PCS syndrome.
We studied immortalized fibroblast cells from patients 1 and 2 for the expression of Mad2, Bub1, Bub3, BubR1, and p55cdc proteins, and the mode of immunofluorescence staining of metaphases with antibodies of these proteins and CENP-B, -C, -E, and -F. Of these proteins studied, only BubR1 showed both reduction of protein expression (>50%) and diminished kinetochore signals. Kinetochore signals of Mad2, Bub1, and CENP-E, were not altered. The expression level of p55cdc was normal but its signal intensities in the kinetochore were markedly decreased (Fig. 4D). Microcell-mediated transfer of chromosome 15 containing the BUB1B locus restored kinetochore association of p55cdc. These results suggest that in these patients the reduced expression of BubR1 did not alter kinetochore localization of Mad2, Bub1, and CENP-E, but caused abolished kinetochore signals of p55cdc.
In conclusion, cells in the patients with the PCS syndrome show >50% decrease in expression or activity of BubR1. This results in markedly reduced recruitment of p55cdc to the kinetochore in mitosis and abnormal mitotic spindle checkpoint.
Acknowledgements
We thank the families for their cooperation in the study, Drs. H. Numabe, Y. Tsuji, Y. Sugio, M. Tsukahara, A. Asamoto, T. Takumi, and M. Kawamura for their help in collecting the samples, Dr. H. Masumoto for the antibodies to CENP-B and CENP-C, and Mses. T. Jo, A. Kamesako, and H. Ikeda for assistance.




