Karyotype–phenotype analysis and molecular delineation of a 3p26 deletion/8q24.3 duplication case with a virtually normal phenotype and mild cognitive deficit
To the Editor:
The terminal ends of most human chromosomes are G-band negative and are usually gene-rich, containing a higher than average density of genes [Niimura and Gojobori, 2002]. Microscopically discernible deletions of the terminal band of a chromosome are usually associated with significant clinical abnormality. For example, chromosome 4p deletion (Wolf-Hirschhorn syndrome) and chromosome 5p deletion (cri du chat syndrome) have critical regions in the most distal 4p16 and 5p15 band, respectively [de Grouchy and Turleau, 1984]. Since the terminal band of 3p26 is an exception as it is G-band positive, one can predict that a deletion of 3p26 might be accompanied by milder clinical symptoms than those resulting from deletion of the terminal bands of other chromosomes. We report here clinical findings in a boy with concurrent 3p26 deletion and 8q24.3 duplication, whose only clinical symptom is deficient cognitive skills. We suggest that his reduced cognitive skills are more likely the result of duplication of genes on terminal 8q, rather than deletion of genes on terminal 3p.
Our patient was born at term after an uneventful pregnancy with a birth weight of 3,450 g and a length of 50 cm. His developmental milestones were normal. He sat at 6 months, crawled at 9 months, and walked independently at 14 months. Psychological testing (Stanford Binet Intelligence Scale) at 3 years of age revealed a composite IQ score of 97 (range 91–103). Subsequent developmental assessments, in conjunction with his enrollment in kindergarten, showed a definitive delay in cognitive skills, speech and language skills, and fine motor skills. He was categorized as multiply disabled. His Peabody Picture Vocabulary Test III scores were at the 39th centile at 4½ years and the 35th centile at 5½ years. He scored solidly in the 36–41 months range but had some scattered scores in the 42–47 months range of the test. He was first seen at the genetics clinic at 5½ years of age. He was quiet, but answered simple questions himself. He had occasional staring spells and absent-mindedness. A seizure disorder was ruled out. He wore glasses for hypermetropia. He was not able to write his own name. His weight and height were between 25th and 50th centile, and head circumference was at the 75th centile. No dysmorphic feature was noted. A year later, the staring spells subsided. He needed extra help in kindergarten and was placed in a special education class in the first grade. A chromosome study was subsequently requested to rule out the possibility of a sex chromosome aberration. He was last seen at 9½ years of age; he remained a friendly and enthusiastic boy, but had become more talkative over the years and stuttering had become apparent. He continued to receive physical, occupational, and speech/language therapies.
Cytogenetic Study
The only cytogenetic abnormality detected was a small terminal aberration of distal 3p [Fig. 1a]. The subsequent chromosomal studies on the parents showed that his father had the same 3p aberration. Further scrutiny of the father's karyotype revealed that he had an apparently balanced translocation between 3p and 8q [Fig. 1b] with breakpoints at 3p25.3 and 8q24.3 [Fig. 1c]. The karyotype of his mother was normal. A FISH study using whole chromosome 8 painting probe (wcp8 probe from Vysis, Inc., Downers Grove, IL) indicated a small amount of chromosome 8 material translocated to the tip of the short arm of one chromosome 3, and a small portion of the terminal long arm of chromosome 8 that remained unstained. A subtelomeric FISH study using the Cytocell Chromoprobe Multiprobe-T System showed that the subtelomeric probes D3S4559 and D8S1925 were reciprocally translocated in the father, while the patient had lost one copy of D3S4559 and gained an extra copy of D8S1925.
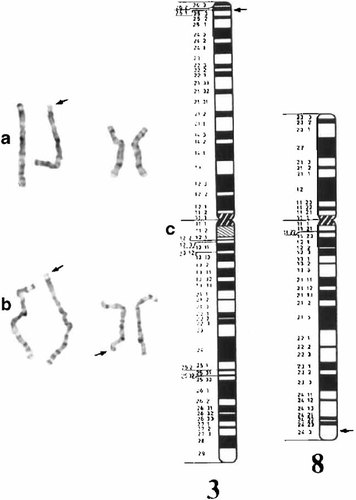
a: Partial karyotype of the patient showing chromosome pairs #3 and #8. The arrow denotes the apparently deleted 3p. b: Partial karyotype of the father showing chromosome pairs #3 and #8. The arrows denote a small reciprocal translocation between terminal 3p and terminal 8q. c: Idiogram of chromosomes 3 and 8 at around 850 band stage. The arrows denote the most likely breakpoints according to the cytogenetic interpretation.
Molecular Genomic Study
As previously described [Shrimpton et al., 1999], more precise translocation breakpoints were determined by performing microsatellite marker analysis using polymorphic DNA markers on the proband and his parents. Microsatellite markers were selected based on their location in the regions of interest and their degree of heterozygosity. The breakpoint on chromosome 3p was mapped to the most proximal region of the band 3p26.1 rather than in the 3p25.3 band as predicted by the karyotype. The breakpoint was localized between the more distally placed marker D3S1304 [deleted] and the more proximally placed D3S3591 [not deleted] on 3p26.1. The distance between these two markers is 1.27 Mb, and the deletion is between 6.89 and 8.16 Mb in size. The gene responsible for Von Hippel-Lindau syndrome is located about 2 Mb further proximal from D3S3591 and thus can be assumed not to be deleted. The precise breakpoint on 8q was between the more proximally placed marker D8S1050 [not duplicated] at 8q24.23 and the more distally placed D8S1717 [duplicated] at 8q24.3, and thus involved a duplication of between 4.77 and 6.68 Mb in size [Table I]. Since D8S1050 is closer [0.3 Mb] to 8q24.3 than D8S1717 is to 8q24.23 [1.6 Mb], it is more likely that the breakpoint is in 8q24.3 rather than 8q24.23.
| Father | Patient | Mother | Result | Cytogene. (band) | Marshfield (cM) | Ensembl (Mb) | |
|---|---|---|---|---|---|---|---|
| Chromosome 3 (locus) | |||||||
| Telomere | 0 | ||||||
| D3S4559 (subtelomeric FISH) | Translocated | Deleted | Normal | p26.3 | 1.32 | 0.23 | |
| D3S3630 | 12 | 2 | 22 | Uninform. | p26.3 | 10.7 | 2.67 |
| D3S1620 | 21 | 1 | 12 | Uninform. | p26.2 | 14.46 | 3.47 |
| D3S1560 | 32 | 2 | 24 | Uninform. | p26.1 | 18.97 | 4.02 |
| D3S1304 | 44 | Δ1 | 11 | Deleted | p26.1 | 22.33 | 6.89 |
| D3S3728 | 42 | 2 | 21 | Uninform. | p26.1 | 24.29 | |
| D3S3591 | 13 | 31 | 12 | Not deleted | p26.1 | 24.89 | 8.16 |
| GATA164B08/D3S4545 | 18 | 87 | 77 | Not deleted | p25.3 | 26.25 | |
| Centromere | 91.6 | ||||||
| Centromere 8 (locus) | |||||||
| Centromere | 45.1 | ||||||
| D8S1753 | 12 | 22 | 21 | Not duplic. | q24.23 | 154.02 | 136.69 |
| D8S502 | 23 | 31 | 13 | Not duplic. | q24.23 | 154.02 | 137.44 |
| D8S274 | 23 | 35 | 54 | Not duplic. | q24.23 | 154.02 | 137.3 |
| D8S1761 | 22 | 21 | 12 | Uninform. | q24.23 | 154.02 | 137.52 |
| D8S1050 | 35 | 52 | 21 | Not duplic. | q24.23 | 139.62 | |
| D8S1741 | 32 | 23 | 31 | Uninform. | q24.3 | 162.94 | 140.62 |
| D8S1743 | 52 | 25 | 51 | Uninform. | q24.3 | 162.94 | 140.64 |
| D8S1717 | 35 | 354 | 42 | Duplicated | q24.3 | 164.25 | 141.53 |
| D8S1836 | 21 | 12 | 22 | Uninform. | q24.3 | 165.93 | 143.81 |
| D8S373 | 34 | 341 | 13 | Duplicated | q24.3 | 164.47 | 144.33 |
| D8S1925 (subtelomeric FISH) | Translocated | Duplicated | Normal | 167.9 | |||
| Telomere | 146.3 |
- Results of subtelomeric FISH studies and microsatellite marker analyses are shown; cytogenetic band positions and locations of loci according to the Marshfield map and the Ensembl Genome Database (version 19.34a.1 of December 15, 2003) are included. Bolded results indicate the interval in which chromosomal breakpoints are found. The delta symbol denotes a deletion. The Von Hippel-Lindau locus is found at 3p25.3 at 10.16 Mb on the Ensembl map.
The karyotype of our proband suggested a loss of the entire 3p26 band and a gain of some or all of the 8q24.3 band. The molecular analysis confirmed an almost total deletion of the 3p26 band, and that the duplication of 8q24.3 band may not be total. Thus, the molecular analysis essentially agreed with, and refined, the cytogenetic interpretation. This karyotype raises the question as to whether the clinical feature of our patient, that is, mild cognitive deficit, was caused by the 3p26 deletion, by the 8q24.3 duplication, or both.
Over 30 cases of distal 3p deletion are known in the literature [among others Verjaal and de Nef, 1978; Higginbottom et al., 1982; Nienhaus et al., 1992; Benini et al., 1999; Kariya et al., 2000]. The clinical features associated with 3p deletion syndrome are psychomotor retardation, pre-/post-natal growth retardation, microcephaly, postaxial polydactyly, blepharoptosis, plus a few other less frequent signs. Narahara et al. [1990], Phipps et al. [1994], and Drumheller et al. [1996] showed that the critical region responsible for the 3p deletion syndrome was in the 3p25.3 band. As our patient did not have the typical clinical features of 3p deletion syndrome, we explored the possibility that the deletion of the p26 band could be compatible with a normal phenotype. Knight et al. [1995] described del(3)(p25.3) in a normal mother and her normal infant in conjunction with a prenatal diagnosis for advanced maternal age. The poster presentations of Jervis et al. [2002] and Sklower-Brooks et al. [2002] confirmed the notion that deletion of the G-positive 3p26 band could be compatible with a virtually normal phenotype. Although further detailed molecular genomic studies were not performed in these three cases and the breakpoints were considered to be at 3p25.3, the critical region responsible for 3p deletion syndrome on 3p25.3 may not have been involved in these three families. Thus, almost total deletion of 3p26 band in our patient may not be the basis of his cognitive deficit. Based on the microsatellite analysis, we were able to further conclude that our patient was not haploinsufficient for the Von Hippel-Lindau gene, which is located on the 3p25.3 and over 2 Mb proximal from the breakpoint in 3p26.1.
It is difficult to find a pure 8q24.3 duplication case in the literature. Eussen et al. [2000] reported a patient with concurrent 16p13.3 deletion and 8q24.3 duplication, whose clinical features were dominated by the features of the 16p13.3 deletion, manifesting in tuberous sclerosis, alpha thalassemia trait, and polycystic kidney disease. Bijlsma et al. [1999] reported five patients from a large family with a cryptic translocation between 2q37.3 and 8q24.3, who showed a 2q37.3 deletion and clinical features resembling Albright hereditary osteodystrophy (AHO). These patients had moderate to severe mental retardation. Since almost all patients with terminal deletion of the 2q37.3 band and AHO phenotypes have mild mental retardation, the more severe mental deficit in Bijlsma et al. patients was attributed to the concurrent small 8q24.3 duplication. It is therefore reasonable to suggest that a duplication of 8q24.3 is associated with mild cognitive deficit, and that the cognitive deficit in our patient was likely attributed to the duplication of the terminal 8q24.3 band.
The terminal ends of chromosomes are usually G-band negative, appearing pale in G-banding pattern, and usually containing many significant genes. An excellent summary on this subject is found in Niimura and Gojobori [2002]. It appears, however, that the terminal end of the short arm of chromosome 3 is an exception from the above generality, being G-band positive and staining darkly in its G-banding pattern. There are only about a dozen known genes mapped to within the terminal 8 Mb of this chromosome arm, distal to D3S3591, representing a low gene density [http://genome.ucsc.edu/cgi-bin/hgGateway]. This may account for why deletion of 3p26 results in a lack of significant phenotypic consequences. Typically, deletions are associated with more severe clinical symptoms than duplications of the same chromosome segment. Thus, it can be postulated that a pure duplication of the 3p26 band, like its deletion, is also likely to have a mild phenotypic effect. In contrast, the duplicated region on the 8q chromosome, distal to D8S1050, is gene-rich, particularly the terminal 2.5 Mb. Thus, an additional copy of many genes is present in our patient, one or more of which could cause the cognitive deficit. Even though the rearrangement in our patient was easily detectable by a routine cytogenetic examination, other terminal rearrangements may only be detected by a subtelomeric FISH study using commercially available probes, such as Chromoprobe-Multiprobe T System of Cytocell or the Vysis system.




