The natural history of severe anemia in cartilage-hair hypoplasia
Abstract
Anemia is seen in over 80% of patients with cartilage-hair hypoplasia (CHH). While this is usually mild and self-limited, some patients demonstrate a severe, persistent anemia resembling that seen in Diamond-Blackfan anemia (DBA). This paper examines the natural history of 12 patients with CHH and severe anemia. Phenotypic features and mutation data (where available) were reviewed, but no significant differences were found that predicted severe anemia. Severe anemia is estimated to occur in approximately 6% of CHH patients and is permanent in more than half of these patients. Thrombocytosis, though not previously reported in CHH, was noted in five patients, similar to that seen in DBA. The role of possible gene–gene and gene–environment interactions is discussed. © 2005 Wiley-Liss, Inc.
INTRODUCTION
Cartilage-hair hypoplasia (CHH) [OMIM 250250], an autosomal recessive chondrodysplasia, was first described in 1965 in the Old Order Amish [McKusick et al., 1965]. It has subsequently been found in other populations, particularly in Finland [Kaitala and Perheentupa, 1980]. Cardinal features include a metaphyseal chondrodysplasia resulting in short-limbed short stature; fine, sparse hair; Hirschsprung disease; and T-cell immune deficiency with lymphopenia [McKusick et al., 1965; Mäkitie and Kaitila, 1993]. Additional hematologic abnormalities are seen including anemia and neutropenia [Mäkitie et al., 1992, 1998]. Mutations in the gene coding for the RNA subunit (RMRP) of the mitochondrial RNA processing endonuclease (RNase MRP) are responsible for CHH [Ridanpää et al., 2001].
Anemia is seen in as many as 73% of CHH patients [Mäkitie et al., 1992]. The anemia is most frequently macrocytic and never microcytic [Mäkitie et al., 1992]. The anemia likely results from defects in cell proliferation and/or maturation, as impaired colony growth of erythroid precursors has been noted [Juvonen et al., 1995]. In most cases, the anemia is mild and resolves spontaneously during early childhood [Mäkitie et al., 1992]. In more severe cases, the anemia resembles that seen in Diamond-Blackfan Anemia (DBA), [OMIM 205900; Mäkitie et al., 1992]. This report describes the natural history of severe anemia in a group of CHH patients. Our observations suggest that in many cases the anemia is persistent and often has a poor prognosis.
PATIENTS AND METHODS
The study included patients with an established diagnosis of CHH and a history of severe anemia. In addition to patients presently under follow-up at Gundersen Lutheran Medical Center, Wisconsin (Patient #1), the Helsinki University Hospital, Finland (Patients #2 and 3), and the Karolinska University Hospital, Sweden (Patient #4), patients were identified through the Finnish CHH database (Patients #5–9) and from a review of the medical literature (Patients #10–12). The diagnosis of CHH was based on the typical clinical and radiographic characteristics [McKusick et al., 1965] in all cases and was confirmed by mutation analysis of RMRP [Ridanpää et al., 2001] in seven cases. Severe anemia was defined as hemoglobin (Hgb) <3.0 g/dl at presentation or a history of repeated red blood cell transfusions. Clinical, biochemical, and genetic data were collected from hospital records, the Finnish CHH database, or from publications for each patient. Patient information is summarized in Table I.
| Patient | Sex | Age | Lowest Hgb (g/dl) | MCV (fl) | WBC (× 109/L) | Platelet (× 109/L) | Trans/BMTa | Height (SD) | Mutation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 9 m | 2.1 | 101.6 | 4.6 | 574 | Y | −6 | 70A > G × 2 |
| 2 | M | 4 y | 5.3 | 83 | 3.1 | 1,255 | Y | −9.7 | 70A > G × 2 |
| 3 | F | 7 y | 3.4 | 86 | 2.2 | 181 | Y | −9.9 | 70A > G × 2 |
| 4 | M | 2 y | 5 | Normal | Low | High | Y | −5.5 | 70A > G/35C > T |
| 5 | F | 13 y | 3 | 89 | 5.9 | 251 | N | −8.8 | 70A > G × 2 |
| 6 | F | 2 wc | 6.9 | 93 | 1.9 | 11 | Y | −6.2 | ? |
| 7 | F | 3 mc | 2.9 | ? | ? | ? | Y | −6 | ? |
| 8 | F | 6 mc | 5.5 | ? | ? | ? | Y | −8 | ? |
| 9 | M | 40 y | 3.6 | 88 | ? | ? | N | −8 | 70A>G × 2 |
| 10 | M | 11 m | Hct 19% | ? | <1b | ? | Y | <3rd centile | ? |
| 11 | M | 4.5 y | 4.7 | 106 | 11.1 | 800 | Y | −7.5 | ? |
| 12 | F | 8 y | 2 | 79 | 1.7 | 483 | Y | −8 | 195insT/63C > T |
- y, years; m, months; w, weeks.
- a Y means either persistent transfusion requirement or BMT.
- b Lymphocyte count, not total white count.
- c Age at death.
RESULTS
Twelve patients meeting the entry criteria were identified. In all, but one case, the anemia developed before 5 months of age; Patient #12 developed aplastic anemia at 2½ years. In three patients (30%), the outcome was fatal; the Patients (# 6, 7, and 8) died at 2 weeks, 3 months, and 6 months, respectively. Median follow-up for the living patients was 4½ years (range 9 months–40 years).
Patient #1
The patient is an Amish boy who was referred at 2 months of age to one of the authors (M.S.W) for severe anemia, (Hgb of 2.1 g/dl). He was the term product of an unremarkable 10th pregnancy and home delivery. No unusual bleeding was noted at the time of delivery. He was eating well and developing normally. At 2 months, a visiting nurse noted that he was pale and he was referred to a local physician. Anemia was noted at that time. The family history was negative for short stature or significant anemia.
On examination at 2 months, the patient was afebrile, in no distress, and eating well. His weight was 3.5 kg (just <3rd centile), length 45 cm (−6.0 SD), and OFC 37 cm (10th centile). Pertinent physical findings included rhizomelic shortening of the limbs, with full range of motion of all joints; distal digital hypoplasia and short nails, and sparse, fine, curly hair. Pertinent negatives on examination included normal craniofacial exam; no jaundice or scleral icterus; no organomegaly, and normal neurologic exam.
CBC at 2 months showed a Hgb of 2.1 g/dl (lower limit of normal for age 10.5 g/dl), MCV 101.6 fl (normal upper limit for age 88 fl), WBC 4.6 × 109/L (lower limit of normal 5.0 × 109/L), and platelet count of 574 × 109/L (upper limit of normal 385 × 109/L). The differential white count showed a normal absolute neutrophil count of 3.02 × 109/L but a low absolute lymphocyte count of 1.15 × 109/L (lower limit of normal 2.05 × 109/L). Platelet and red cell morphology was normal with the exception of red cell macrocytosis. Reticulocyte count was markedly low for the degree of anemia (37.2 × 109/L). Bilirubin was normal. Skeletal survey showed foreshortening of all limbs with rhizomelia and mesomelia. There was prominent bowing of both femora as well as metaphyseal cupping and slight lipping of the proximal femora (Fig. 1). A clinical diagnosis of CHH was made.
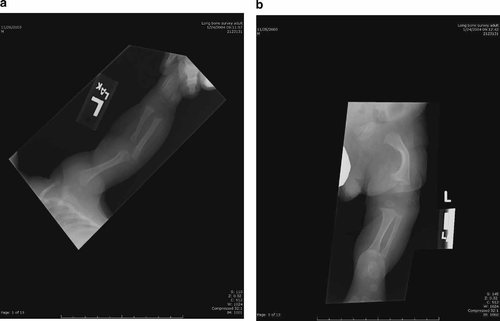
a, b:. Radiographs Patient #1. Note generalized shortening of the long bones, significant curvature of the femur, and subtle metaphyseal changes.
The patient was slowly transfused with irradiated packed red blood cells and discharged to home. He required another transfusion at 3 months of age, but the Hgb subsequently stabilized at 10 g/dl until 9 months of age, when the Hgb dropped to 4.5 g/dl with a very low reticulocyte count of 3.9 × 109/L. The patient was again transfused and has remained transfusion-dependent. Immune studies have not been done to date; clinically there are no signs of compromised immunity. Analysis of the RMRP gene demonstrated homozygosity for an A > G transversion at nucleotide position 70, confirming the diagnosis of CHH.
Patient #2
The patient is a 4-year-old boy. He was the 32-week product of a first pregnancy of healthy, unrelated Finnish parents. The diagnosis of chondrodysplasia was suspected prenatally based on shortened long bones seen on ultrasound. Birth Hgb was 8.6 g/dl. He developed severe respiratory distress syndrome (RDS) requiring ventilatory support. While the RDS resolved, the patient has persistent anemia requiring red blood cell infusions every 2–4 weeks. The lowest measured Hgb was 5.3 g/dl at 4 months of age. At that time, he had thrombocytosis (1,255 × 109/L) but a normal white count (5.8 × 109/L). The reticulocyte count was 3.1% at 4 months of age, but subsequently declined to 0.3%, where it has remained. Hemolysis is not present. Bone marrow examination has consistently shown marked erythroid hypoplasia without morphologic changes. Bone marrow stem cell cultures have consistently shown poor growth of erythroid as well as megakaryocyte lineages. At 4 years of age, his hematologic evaluation showed a normocytic anemia (Hgb 7.2 g/dl, MCV 83 fl), leukopenia (WBC 3.1 × 109/L), and normal platelet count (386 × 109/L). The transfusions have resulted in iron overload with serum ferritin of 2,450 ng/ml (normal for males 12–300 ng/ml) and transferrin saturation of 95% (males <60%).
He has impaired T-cell function resulting in recurrent respiratory infections. The height z-score has ranged from −9.7 to −8.0. Growth hormone testing with arginine resulted in subnormal response with peak value of 8.2 mU/L (normal >20 mU/L) and growth hormone treatment has been considered. The patient is homozygous for the 70A > G transversion in the RMRP gene.
Patient #3
The patient is a 7-year-old girl. She was born to healthy unrelated Finnish parents at term. Her initial Hgb was 3.4 g/dl. She required red blood cell transfusions until 6 months of age. The reticulocyte count was 3.1% at the age of 3 months but later stabilized at 0.5%. No hemolysis was present. Initial bone marrow aspirate at 3 months of age showed normal cellularity, including a normal amount of, but morphologically megaloplastic, erythrocyte precursors. At 6 months of age, there was apparent spontaneous recovery until the age of 18 months, when the patient again developed severe anemia requiring red cell transfusions every 2–3 weeks. There was no response to treatment with corticosteroids. The transfusions have resulted in severe iron overload (serum ferritin 5,640 ng/ml [normal female 12–150 ng/ml], transferrin saturation 99% [females <50%]). A liver biopsy at 2 years of age showed grade III hemosiderosis, but no clinical signs of iron overload have been noted. Repeat bone marrow examinations showed erythrocyte precursors within the normal range until 21 months, when erythroid hypoplasia became evident. In stem cell cultures, erythroid growth has been extremely poor, even during the time of apparent recovery. Bone marrow transplantation (BMT) is under consideration.
She has combined immune deficiency and has been on intravenous immunoglobulin since 6 months of age. The patient has been on growth hormone treatment since 13 months of age, despite normal growth hormone response to arginine. Her height z-score has increased from −9.9 to −6.0. The patient is homozygous for the 70A > G transversion in the RMRP gene.
Patient #4
The patient is an almost 2-year-old boy. He was born prematurely after 34 normal gestational weeks. The birth weight was 1,845 g and length 40.6 cm. He was the second child of non-related parents from Sweden. The father has ancestors from Finland and the mother from the Netherlands. The neonatal examination was remarkable for short limbs. At 1 month of age, he was referred for evaluation of fatigue and anemia (Hgb 5.0 g/dl). The anemia was normocytic and slightly hyperchromic. The total white blood count was low, and the differential showed lymphopenia. The platelet count was high. There were no sign of hemolysis. Antibody titers against CMV, EBV, and parvovirus were negative. PCR analysis for parvovirus B19 (PVB19) and human herpes virus 6 (HHV6) DNA from bone marrow samples was negative. Length was −3 SD and weight −1 SD. DBA was considered due to anemia, thrombocytosis, and low reticulocytes. Bone marrow examination revealed an active marrow but a decreased erythropoiesis without dysplastic signs and that could be consistent with DBA. X-ray of the whole body skeletal showed a skeletal dysplasia.
An immunologic work-up was performed. Levels of immunoglobulins A, M, G, and the subclasses of IgG were normal. However, he has low levels of CD3+ T lymphocytes, slight decreased levels of CD19+ B-cells, but normal amount of NK-cells. In blood samples from the patient, there were signs of T-cell defects with an in vitro low response to anti-CD3, low production of interferon on mitogen stimulation, and decreased helper T-cells to B-cells. Clinically, the lymphopenia and the T-cells defect have not caused a problem as he attends day care without severe or recurrent infections.
He was initially transfused with blood. At 5 months of age, he was treated with prednisolone 2 mg/kg/day and the Hgb increased from 7.5 to 10 g/dl. Prednisolone was tapered over the next several months. For almost 10 months the Hgb was stable at 9.0 g/dl. The Hgb level then dropped to 6.7 g/dl. Prednisolone was restarted and the Hgb increased above 10.0 g/dl. The steroids were again tapered, due to concern about his immune deficiency and slow growth (length at 1.8 years was −5.5 SD). The Hgb level fell to 7.0 g/dl. A third course of prednisolone was initiated, but this time there was no response. He is now transfusion-dependent. The erythropoietin level in serum is 100 times above the normal reference level. The reticulocytes count is usually low, but occasionally normal or slightly increased.
In course of the diagnostic evaluation, analysis of several genes was undertaken. No mutations were found in FGFR3, RPS19, or SHOX. Analysis of the RMRP gene (HUCH Laboratory Diagnostics, Finland) identified two mutations: a 35C > T transversion (paternal) and a 70A > G transversion (maternal). This confirmed the diagnosis of CHH.
Patient #5
The patient is a 13-year-old girl. Her Hgb at birth was 3.0 g/dl. The MCV is not available. Leukopenia and thrombocytopenia were reported. The patient was transfusion-dependent until age 5 months, at which time she experienced spontaneous and apparently permanent recovery. At 13 years of age, her hematologic parameters are: Hgb 11.9 g/dl, MCV 89 fl, WBC 5.9 × 109/L, platelet 251 × 109/L. Her height z-score has ranged between −7.4 and −8.8. She is homozygous for the 70A > G transversion in the RMRP gene.
Patient #6
This girl was born at full term with significant short stature and rhizomelia (length −6.2 SD). She had anemia at birth (Hgb 11.0 g/dl, WBC 21.3 × 109/L, platelet 164 × 109/L) and required several transfusions. She subsequently developed pancytopenia and cardiac failure and died at age 2 weeks. Antibiotics were administered but repeated cultures prior to and during antibiotic treatment failed to grow bacteria. No DNA was obtained for mutational analysis.
Patient #7
This girl died at age 3 months of respiratory failure. Her Hgb at birth was 5.1 g/dl. She was transfusion-dependent for her 3 months of life. At age 3 months, her Hgb was 2.9 g/dl. The birth length was five standard deviations below the mean, and length at death was six standard deviations below the mean. Mutation analysis was not performed. She has a sister with typical CHH but no anemia.
Patient #8
This girl died at 6 months of age. Her birth Hgb was 10.4 g/dl. She was transfusion-dependent for her 6 months of life. The lowest measured Hgb was 5.5 g/dl at age 2½ months. Her birth length was eight standard deviations below the mean. Mutation analysis was not performed.
Patient #9
The patient is a 40-year-old man. His Hgb at 6 months of age was 3.6 g/dl. He was transfusion-dependent until age 1 year, at which time spontaneous permanent recovery occurred. His adult Hgb was 14.6 g/dl, with an MCV of 88 fl. His final adult height is eight standard deviations below the mean. He is homozygous for the 70A > G transversion in the RMRP gene.
Patient #10
The patient is a 11-month-old boy [Wilson et al., 1978]. He was born to parents of North Carolina Lumbee Indian descent. Anemia was noted at birth (Hematocrit 19%) and there were “no reticulocytes.” A bone marrow aspirate revealed decreased erythrocyte precursors, with other marrow elements being normal. Daily prednisone was administered and seemed to reduce the need for transfusion, but this was discontinued at 5 months of age because of an exposure to varicella. He subsequently required repeated transfusions. The MCV was not given.
His length was much less than the third percentile (actual value not reported, but described as much more severe than his affected sister). Additional features included Hirschprung disease, bilateral inguinal hernias, combined immune deficiency, and recurrent infections.
The patient's affected sister has not had anemia.
Patient #11
He is a 4½-year-old boy [Harris et al., 1981]. His parents were Amish. At 2 months of age, severe anemia developed requiring bi-monthly transfusions. No evidence of hemolysis was found. At 21 months of age, the patient had a macrocytic anemia (Hgb 4.7 g/dl, MCV 106 fl), normal white cell count (11.1 × 109/L), and thrombocytosis (800 × 109/L). Bone marrow aspirate showed markedly depressed marrow erythroid precursors. Treatment with oral prednisone resulted in prompt reticulocytosis with a rise in Hgb to 9.3 g/dl within 2 weeks. The Hgb subsequently stabilized at 10.0 g/dl on a dose of prednisone of 0.5 mg/kg/day.
The patient developed lymphopenia by 25 months of age (absolute lymphocyte count of 1.267 × 109/L) with only 10% E-rosette positive cells (normal 50–80%). In vitro stimulation studies were also abnormal.
At 25 months of age, his length was seven and one-half standard deviations below the mean.
Patient #12
She is a 8-year-old girl [Kuijpers et al., 2003]. She was initially diagnosed with kyphomelic dysplasia and the diagnosis of CHH was considered on re-evaluation at age 8. She developed aplastic anemia at age 2½ years. Her hematologic parameters prior to BMT were: Hgb 2.0 g/dl, MCV 79 fl, WBC 1.7 × 109/L, and platelet 483 × 109/L. Her height at age 2½ years was four standard deviations below the mean. The patient was transfusion-dependent until age 3 years, when she underwent allogenic BMT. Her hematologic parameters normalized following BMT. Her height at age 8 years is eight standard deviations below the mean. Analysis of the RMRP gene identified two mutations: 195insT and 63C > T.
DISCUSSION
Severe anemia requiring transfusion is seen rarely in CHH. Prevalence can be estimated at about 6%, based on 7 severe cases out of a total of 114 patients in the Finnish CHH database. The presented cases suggest that the natural course of severe anemia in CHH is variable but often has a poor prognosis. Only two patients showed spontaneous and apparently permanent recovery (Patients #5 and 9). Patient #3 had a temporary recovery for approximately 1 year from 6 to 18 months followed by persistent transfusion-dependent anemia. Patient #1's anemia briefly stabilized but ultimately the patient required additional transfusions. Three patients (#6, 7, and 8) died at a young age, so information about the natural history of their anemia is incomplete. However, it is likely that anemia was a contributing factor in their deaths. It can be concluded from the data presented that roughly one-half to two-thirds of CHH patients presenting with a severe anemia will require lifelong transfusions or BMT, while the rest will show spontaneous permanent recovery. Two patients had relapses after apparent recovery, so patients with remission should be followed to ensure the recovery is, in fact, permanent. More patients will need to be followed for longer time periods to refine these estimates. No clinical features or hematologic parameters were identified that allowed discrimination between those with permanent anemia and those with spontaneous recovery.
The relationship between height and severity of anemia in CHH has been previously noted [Mäkitie et al., 2000]. All of the described patients had severe short stature at or below the reported median height for patients with CHH [Mäkitie and Kaitila, 1993]. Correlation with immune deficiency phenotype was not possible because the majority of the patients had not had a formal immunologic evaluation. However, the presence of severe combined immune deficiency in two of the patients suggests that patients with severe anemia may be more likely to present with not only T-cell but also B-cell-mediated immune deficiency, although Patient #4 had isolated T-cell abnormalities without clinical evidence of immune deficiency.
Patients (5 of the 12) had thrombocytosis on at least one measurement (defined as platelet count >400 × 109/L). Thrombocytosis has not been previously reported in CHH. Thrombocytosis is seen in some patients with DBA [Willig et al., 2000]. Given that DBA is the principle hematologic diagnosis that needs to be distinguished from CHH-associated anemia, it is important to recognize that thrombocytosis is present in both conditions, and is therefore, not useful in distinguishing between the two conditions.
One Finnish patient was treated with steroids without response (Patient #3). Patient #4 initially responded to prednisolone, but this response was lost over time. Patient #10 may have had a partial response to prednisone, but this was discontinued due to concerns about compromise of immune function. Patient #11 apparently responded to steroid therapy without adverse immunologic effects. This was tried empirically because 72% of DBA patients showed an initial response to steroids [Ball et al., 1996]. Given that some patients with CHH undergo spontaneous recovery, it cannot be said with certainty that the response in Patients #4, 10, and 11 was due to the steroids. Whether a subset of CHH patients with severe anemia would respond to steroids, is unknown. The possible benefits of the treatment should be carefully weighed against the potential side effects in a condition associated with immune deficiency and short stature.
The pathogenesis of the severe anemia phenotype is not understood. Genetic heterogeneity can be ruled out, as five of seven patients who underwent mutation analysis were homozygous for the 70A > G transversion in the RMRP gene, which is the major mutation among the Finnish and Amish CHH patients [Ridanpää et al., 2003]. In addition, the affected sibs of Patients #7 and 10 were discordant for anemia.
Gene–environment interactions have not been systematically investigated. In particular, PVB19 has been associated with aplastic crises in other hematologic conditions, such as sickle cell anemia [Brown and Young, 1995]. There is a single case report of an apparent relapse of anemia in a patient with DBA that was ultimately attributed to primary infection with PVB19 [Tchernia et al., 1993]. PVB19 infection has also been misdiagnosed as DBA [Heegaard et al., 1996]. Of critical importance in patients with CHH, is the recognition that some immunocompromised patients can develop persistent infection with PVB19 that can cause severe, tranfusion-dependent anemia [Brown and Young, 1995]. This has been seen in patients with primary T-cell immune deficiencies, such as AIDS. This condition is treatable with intravenous immunoglobulin. Patient #3 in the series has been treated with IVIG without resolution of the anemia. PVB19 studies have not been done in this patient. Case #4 is the only CHH patient formally studied for PVB19 infection, although he was studied at presentation, not after the relapses. Given that this is a treatable condition, PCR for PVB19 DNA is reasonable in CHH patients with anemia, particularly patients such as #1, 3, and 4 who have suffered an apparent relapse. Table I.
Gene–gene interactions have been previously postulated as an explanation for the correlation between severity of anemia and height. Mäkitie et al. [2000] observed a correlation between the degree of anemia and levels of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 (IGFBP-3). Erythrocytes have been demonstrated to have receptors for IGF-I and IGF-II [Polychronakos et al., 1983]. Both growth hormone (GH) and IGF-I have been shown to stimulate erythropoesis in vitro [Golde et al., 1977; Claustres et al., 1987]. Mäkitie et al. [2000] did not observe a stimulatory effect in vitro in erythroid precursors from patients with CHH with either GH or IGF-I. One of the patients in the present cohort (Patient #3) has been on GH treatment for 6 years but no improvement in the erythroid aplasia has been noted in bone marrow aspirates, in peripheral blood counts, or in the transfusion requirement. GH stimulation tests have demonstrated normal GH secretion in all tested CHH patients, except Patient #2 in this series [Mäkitie et al., 2000]. Thus, even though GH, IGF-I, and IGFBP-3 play a role in both linear growth and erythrogenesis, no evidence currently supports interaction between RMRP and GH, IGF-I, or IGFBP-3. Similarly, erythropoietin (EPO) levels are elevated in CHH patients, even those with normal Hgb concentrations, ruling out subnormal EPO as the cause of anemia [Mäkitie et al., 2000].
As previously noted, the anemia of Diamond-Blackfan is similar to that of CHH, in that there is a defect of erythrogenesis associated with macrocytosis. Leukocytes and platelets are usually normal, although thrombocytosis has been seen [Diamond et al., 1961]. Short stature is present in 28% of patients and 18% have thumb anomalies [Ball et al., 1996]. Craniofacial anomalies are seen in nearly half the patients [Ball et al., 1996]. Metaphyseal dysplasia has not been reported in DBA. There is marked inter- and intra-familial variability [Sylvester et al., 2004]. About 25% of DBA is due to mutations in the ribosomal protein S19 gene (RPS19) [Draptchinskaia et al., 1999].
Given the similarity between the anemia in CHH and DBA and the fact that all CHH patients and at least 25% of DBA patients have mutations in genes involved in ribosome structure and processing, it is intriguing to speculate about an interaction between the RNase MRP complex and RPS19. This needs to be elucidated in future studies.
Acknowledgements
The authors thank Lars Hagenäs M.D., Ph.D. and Jan-Inge Henter M.D., Ph.D. from the Karolinska University Hospital, Stockholm, Sweden for their assistance with Patient #4.




