Phenotype and X inactivation in 45,X/46,X,r(X) cases
Abstract
We studied a new series of 21 individuals mosaic for a ring X chromosome [r(X)]. Of nine individuals with mental retardation, only one had a r(X) that lacked XIST (X-inactive-specific transcript) and was not subject to X inactivation, which would explain the abnormal phenotype; the remaining eight cases had XIST on their r(X). The majority of cases (five of seven) with mental retardation had an apparently early replicating r(X); but the androgen receptor gene (AR) was methylated on one allele in five of six informative cases, including two cases with an early replicating r(X). These conflicting results on two indicators of X inactivation suggest a potential dissociation between late replication and DNA methylation in these r(X) chromosomes, which may fail to become completely silenced. Of the twelve subjects who were not mentally retarded, all had XIST present on their r(X) and most (8/10) showed a late replicating r(X), together with AR methylation in all five informative cases, indicating r(X) inactivation. Thus, the unusual phenotypic features and mental retardation associated with the presence of a r(X) cannot be explained solely on the basis of presence or absence of XIST. The r(X) in cases with mental retardation were consistently smaller than those in individuals with normal intelligence, perhaps indicating inability for small rings to undergo structural changes associated with complete X inactivation or lethality in cases with a large non-inactivated r(X). Of the Turner syndrome features present in the r(X) cases, only edema was present in a lesser frequency than in 45,X individuals. Our cases generally had a less severe phenotype than those previously reported, suggesting that reported incidences of abnormalities may be influenced by ascertainment bias, with mental retardation potentially unrelated to the presence of the r(X) in some cases. © 2004 Wiley-Liss, Inc.
INTRODUCTION
During normal development, one of the two X chromosomes of mammalian females becomes inactivated [Lyon, 1961]. X-inactive-specific transcript (XIST), a gene located on the proximal long arm of the human X chromosome, is necessary for the initiation of X inactivation and is expressed only from the inactive X [Brown et al., 1991; Avner and Heard, 2001; Plath et al., 2002]. XIST encodes an RNA product that becomes closely associated with the inactive X [Clemson et al., 1996]. Following initiation of inactivation, the X chromosome undergoes a series of changes in its chromatin structure and becomes late replicating. In addition, the CpG islands of X-linked genes become methylated [Brown et al., 1991; Avner and Heard, 2001; Plath et al., 2002]. If both X chromosomes are intact, the choice of which one becomes inactivated is usually random. However, if one X chromosome is structurally abnormal, such as duplicated X, deleted X, isochromosome X, or a ring X chromosome, the abnormal X is typically inactivated in the majority of cells in an adult [Leppig and Disteche, 2001]. These apparently non-random X inactivation patterns probably result from cell selection during embryogenesis [Gartler and Sparkes, 1963; Disteche et al., 1979]. X inactivation skewing in females with an abnormal X chromosome usually results in a Turner syndrome (TS) phenotype, typically associated with a 45,X karyotype.
In contrast to other X chromosome abnormalities, ring X chromosomes can result in a more severe, atypical, phenotype. Whereas the incidence of mental retardation is approximately 10% in individuals with TS [Sybert, 1995], among the subset of individuals with TS who carry a r(X), the incidence is significantly higher (about 30%) [Sybert, 1995]. Previous studies performed on individuals mosaic for a r(X) have demonstrated that many segregate into two well-defined phenotypes correlated with the presence or absence of an intact and functional XIST gene on their r(X) [Duncan et al., 1993; Migeon et al., 1993, 1994; Cole et al., 1994; Wolff et al., 1994; Callen et al., 1995; Jani et al., 1995; Guillen et al., 1997; McGinniss et al., 1997; Dennis et al., 2000; Turner et al., 2000; Matsuo et al., 2000a; Tomkins et al., 2002]. Individuals with physical features typical of TS have often been found to have XIST expressed from their r(X) and to show evidence of inactivation of their r(X). In contrast, individuals with a more severe phenotype that includes mental retardation and congenital malformations atypical for TS may have XIST deleted and/or not expressed from their r(X), preventing normal X inactivation. This more severe phenotype has been attributed to functional disomy for the genetic information present on the r(X) chromosome. However, it has been suggested that the phenotype of r(X) individuals cannot be entirely defined in terms of X inactivation patterns. Indeed, recent studies have confirmed exceptions that cannot be explained by the presence or absence of XIST and/or XIST expression and the observed X-inactivation patterns [Dennis et al., 1993, 2000; El Abd et al., 1999; Kuntsi et al., 2000; Matsuo et al., 2000a; Migeon et al., 2000; Turner et al., 2000; Stankiewicz et al., 2001].
In the present study, we investigated a new series of 21 individuals with a r(X) by a combination of cytogenetic and molecular genetic analyses to characterize the rings. We examined ring size and frequency, presence or absence of XIST, and X inactivation status of the r(X) by replication analysis and androgen receptor (AR) methylation, in relation to the phenotype of our subjects. In addition, the clinical features of our cases were compared to those of 45,X individuals and to the literature.
SUBJECTS
The majority of the subjects (16 of 21 subjects) were from a cohort of 33 individuals who have been followed in a specialty clinic in Seattle for the study of the natural history of Turner syndrome [Sybert, 1995]. Individuals had been referred to the clinic by generalists and specialists of all types, both for initial diagnosis and for management after a diagnosis of TS. No attempt was made to select individuals with unusual features for the clinic or for the purpose of the work reported here. Of a total of 33 girls and women with a r(X), 16 consented to donate blood for this study; 1 died in the newborn period; the remaining 16 either had been lost to follow-up at the time that this study was initiated or declined to participate. Four additional subjects were referred from the practice of one author (J.L.R.) and one from the practice of another author (C.M.C.). Written informed consent (approved by the institutional review boards of the participating centers) was obtained for all subjects.
MATERIALS AND METHODS
Cytogenetic and Fluorescence In Situ Hybridization (FISH) Analyses
Cytogenetic cultures were established from peripheral blood samples using standard techniques. Chromosomes were stained by G-banding. Probes used for FISH analysis included X-whole chromosome paint (wcpX) and XIST (Oncor, Inc., Gaithersburg, MD). A cosmid probe, cIC-61, previously used to define the distal boundary of the human X-inactivation center [Leppig et al., 1993] was provided by H. Willard (Duke University, Durham). A YAC probe for the androgen receptor gene (AR) was provided by A. LaSpada (University of Washington, Seattle). Hybridizations using wcpX and XIST were done following manufacturer's recommendations. In situ hybridization of cosmid probes was done as previously described [Chance et al., 1993]. The post-hybridization temperature used for cIC-61 was 52°C and for AR YAC probe, 49°C. Following hybridization, the slides were stained as described in Edelhoff et al. [1994].
A minimum of 50 metaphase cells were examined after FISH using wcpX to confirm that the ring was completely derived from X chromosome material and to determine the proportion of cells with the ring present. If on initial examination, less than 10% of cells had the r(X) present, a total of 100 metaphase cells were examined to more accurately determine the level of mosaicism. Following FISH using XIST, cIC-61, and AR probes, a total of 20 metaphase cells containing the r(X) were scored for signals whenever possible for each patient.
Replication Studies
The replication status of each r(X) chromosome was defined by bromodeoxyuridine (BrdU) labeling techniques on lymphocyte cultures [Latt, 1973]. After 60–65 hr, BrdU, FudR, and uridine were added to lymphocyte cultures to give a final concentration of 10−4, 4 × 10−7, and 6 × 10−6M, respectively, for approximately 6 hr before the cultures were harvested. Chromosomes were initially stained with Hoechst 33258 (150 µg/ml) and exposed to an ultraviolet light source for 90 min before being stained with a 2% Giemsa solution. By this technique, the late replicating X chromosome was pale in appearance. Whenever possible, we analyzed 20 metaphase cells with the r(X) present to determine its replication status. Slides and photographs were examined and scored by at least two investigators. A case scored as early or late had either an early or a late replicating r(X) in all metaphase cells examined from the sample, and a case scored as early/late had a mixture of cells with early and late replication patterns.
RT-PCR for XIST Expression
Purified RNA was prepared from peripheral blood from the r(X) subjects and from normal male and female controls using a Puregene RNA isolation kit (Gentra System, Inc., Research Triangle, NC), followed by DNAseI (Gibco BRL, Rockville, MD) treatment. After RNA quantification, RT-PCR was performed on 1 µg of RNA. First-strand synthesis was performed on the RNA samples using 200 U of reverse transcriptase (RT) (Gibco BRL) for 60 min at 42°C, followed by 10 min at 92°C, before holding samples at 4°C. XIST primers were from exon 2: AGCTCCTCGGACAGCTGTAA and exon 3: CTCCAGATAGCTGGCAACC with an expected product of 250 bp. PCR reactions were started at 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 59°C for 1 min, and 72°C for 30 sec with a final extension at 72°C for 7 min. Duplicate samples were run for each patient and for a control normal female, with and without RT included. Negative controls included one sample without DNA or RNA added and a female genomic DNA sample without RT added. All products were run on a 1.2% agarose (Nusieve/Seakem) gel, including a 123 bp ladder, and detected by ethidium bromide staining.
Androgen Receptor Methylation
DNA extracted from peripheral blood cells was evaluated for methylation patterns at the human androgen receptor alpha locus (HUMARA) by a modification of the method published by Allen et al. [1992]. Genomic DNA (1 μg) was digested overnight at 37° C in 20 μl volumes containing 20 U HpaII (New England Biolabs, Beverly, MD, 50 U/ml) and/or 20 U HhaI (New England Biolabs, 20 U/ml) and 5 U RsaI (Pharmacia, San Francisco, CA) or RsaI alone. Four microliters of this digest was PCR amplified in 20 μl containing 200 μM dNTPs, 0.5 U AmpliTaq polymerase (Perkin Elmer, Boston, MA), final concentration of 3.7 mM MgCl2 (including MgCl2 from the restriction digest buffer), and 0.4 μM each of the forward primer end-labeled with 0.45 μCi [32P]dATP and unlabeled reverse primer. The following thermal profile was used: initial denaturation for 3 min at 94° C; 30 cycles of 60 sec at 94° C, 45 sec at 62° C and 30 sec at 75° C; and a final 7.5 min incubation at 75° C. Amplified products were electrophoresed for 4 hr at 70 W through 6% polyacrylamide sequencing gels containing 7 M urea and 30% formamide. Dried gels were autoradiographed and the relative band intensities in the RsaI/HpaII/HhaI- and RsaI-digested lanes were compared. Control genomic DNA from a female with complete skewing of X inactivation was included to evaluate completeness of HpaII or HhaI digestion.
RESULTS
Clinical Evaluation
The clinical features of the 21 individuals included in the present study are listed in Table I. Of the 21 cases studied, nine had mental retardation. A subset of features typical of Turner syndrome were observed that included short stature (14/19 cases), edema (4/19 cases), otologic complications (20/21 cases), cardiac (7/17 cases) and renal (6/20 cases) malformations, and thyroid disease (5/18 cases). The reasons for diagnosis (combined in some cases) were short stature in 12 cases, edema in 3 cases, primary amenorrhea in 3 cases, and other abnormalities in 5 cases. Unusual features atypical for TS were seen mainly in those nine girls with mental retardation and included: hypomelanosis of Ito (swirly hypo/hyperpigmentation along the lines of Blaschko) in two of nine cases; syndactyly and/or polydactyly in three of nine cases; and dysmorphic facial features distinct from the facial features of TS in two of nine cases. One case (SA 101) was thought to have features reminiscent of Kabuki syndrome. Syndactyly was also present in one case without MR.
| Casea | Ageb | Heightc | Edema | Otod | Cardiace | Renalf | Thyrg | CNSh | dxi | Mensesj | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SA-114 | 13 | 136, S | +k | om | −k | dup R renal artery | − | MR (severe), ADHD | 4 | P | Intractable diarrhea as infant, syndactyly fingers 3–4, severe vasculitis |
| SA-103 | 25 | 144.2*, S | + | snhl | hbp, mvp | − | + | MR, SB: 54 fs, seizures | 1 | H | Gonadectomy age 12, feeding difficulties, hammer toes |
| SA-101 | 4 | NL | + | om | − | − | unk | MR | 1 | P | Bilateral epicanthal folds, large ears, flattened philtrum, hypertelorism, hypopigmentation, strabismus, clinodactyly, syndactyly |
| SA-96 | 12 | un | − | om, pe, t/a, snhl | asd, mr | dup L kidney | − | MR (severe), macrocephaly, seizures% | 4 | P | Abnormal facial features, swirly pigmentation |
| SA-106 | 30 | 151.8, NL | − | Cholesteoma, mixed snhl, mastoidectomy | unj | − | − | MR (mild), anorexia | 3 | H | In group home |
| SA-121 | 10 | S | − | om, pe | − | − | − | MR, ADD | 2 | P | ? vitiligo |
| SA-111 | 10 | NL | un | om | − | − | − | Borderline MR, 83 vs, 73 ps, 76 fs, ADD | 4 | P | Multiple nevi; Dupuytren's contractures R 3rd finger; epicanthal folds |
| SA-110 | 5 | 95.9, S | − | om | un | Bilateral reflux, nl ultrasound | − | MR | 4 | P | Unilateral polydactyly, bifid thumb, iugrm, nystagmus, esotropia, swirly hypopigmentation, vomiting, and diarrhea |
| SA-122 | 16 | S | un | om, pe, t/a | − | − | − | MR, WISC-R: 58 vs, 64 ps, 57 fs | 2 | H | Bicornuate uterus |
| SA-127 | 2 m | un | − | om, pe | lvh, hbp | Multicystic, dysplastic L kidney s/p nephrectomy; reflux on R | − | Special ed | 4 | P | Severe feeding difficulties, G tube until age 2, sacral dimples, syndactyly toes 2–3 |
| SA-100 | 15 | 145.9, S | − | om, pe, t/a | − | − | − | Normal, high school | 2 | H | Ptosis |
| SA-102 | 47 | 140.3, S | − | om, snhl, ruptured tm | pda | − | − | Normal, WAIS: 112 vs, 83 ps, 99 fs | 2 | H | Ptosis, gallstones, rheumatoid arthritis, psychiatric dx of hysteria |
| SA-109 | 33 | 152.8*, S | − | om, pe, t/a | hbp | − | + | Normal, college grad | 2 | H | Diabetes, colorblindness$ |
| SA-104 | 22 | 146*,#, S | − | om, pe | bcav, ai | Horseshoe | + | ADD$ | 2 | H | |
| SA-105 | 39 | 154.7*, NL | − | om, pe, t/a, snhl | un | − | + | Normal, bank clerk | 3 | H | Colorblindness, polycoria |
| SA-112 | 19 | S | − | om | − | − | un | Normal, 104 vs, 87 ps, 94 fs | 2 | un | Minimal scoliosis |
| SA-113 | 12 | S | − | om | − | Fused kidneys | un | Normal, 117 vs, 95 ps, 107 fs | 2 | P | Short 5th metacarpal, cubitus valgus, hypopigmentation |
| SA-124 | 16 | 154#, NL | − | − | un | − | − | Normal, high school | 2 | S | Elevated fsh/lhl |
| SA-123 | 52 | 142.2*, S | − | T/a, sinusitis | hbp | un | − | Normal, college grad | 2, 3 | H | Pyloric stenosis, depression, myopia, amblyopia, cataracts |
| SA-126 | 13 | S | − | om, t/a | − | − | + | ADD | 2 | P | Normal fsh/lhl, amblyopia$ |
| SA-107 | 10 | S | Mild | om | − | − | − | Normal | 1, 2 | P | Mild syndactyly, toes 2–3 |
- a Case number.
- b Age in years or months (m) at last evaluation.
- c Height in cm when available: *androgens treatment; #human growth hormone treatment; S, short stature, NL, normal height, inferred from specific measurement, and when no measurement available, inferred from referral indication.
- d Otologic complications: om, recurrent otitis media; pe, tympanostomy tubes; t/a, tonsillectomy/adenoidectomy; snhl, sensorineural hearing loss; tm, tympanic membrane.
- e Cardiac abnormalities: hbp, hypertension; mvp, mitral valve prolapse; ai, aortic insufficiency; pda, patent ductus arteriosus; asd, atrial septal defect; mr, mitral regurgitation; lvh, left ventricular hypertrophy; bcav, bicuspid aortic valve.
- f Renal abnormalities: dup, duplicated; R, right; L, left.
- g Thyroid findings: +, thyroid disease; −, no disease.
- h Central nervous system: MR, mental retardation; SB, Stanford–Binet; WISC-R, Wechsler Intelligence Scale-Revised; WAIS, Wechsler Adult Intelligence Scale; fs, full-scale; vs, verbal scale; ps, performance scale; ADD, attention deficit disorder; ADHD, attention deficit hyperactivity disorder; %, s/p subdural effusions in infancy, question of abuse, family history of delays.
- i Reason for diagnosis: 1, congenital edema; 2, short stature; 3, primary amenorrhea; 4, other.
- j H, hormone therapy; S, spontaneous; P, prepubertal.
- k +, present; −, absent; un, unknown; $, family history for same.
- l fsh, follicle stimulating hormone; lh, luteal hormone.
- m iugr, intrauterine growth retardation.
Table II summarizes the incidence of major features found in the large TS clinic population followed by one of us (V.P.S.), which includes a total of 33 r(X) subjects, inclusive of 16 of the 21 cases reported here in detail. Five additional cases listed in Table I (SA 114, SA 101, SA 111, SA 113, and SA 107) were ascertained in other clinics and not included in this comparison, in order to achieve a more unbiased comparison between r(X) and 45,X individuals. Comparison between clinical features of these 45,X/46,X,r(X) cases and 45,X cases showed a greater incidence of mental retardation, and a lower incidence of edema in the r(X) population (Table II). These differences were shown to be highly significant at P = 0.01, using a qui square test.
| Feature | Clinic populationa | Literature reviewb |
Dennis et al. [2000]; Kuntsi et al. [2000] c |
||
|---|---|---|---|---|---|
| Karyotype | 45,X/46,X,r(X) | 45,Xd | 45,X/46,X,r(X) | 45,X/46,X,r(X) | 45,X |
| Mental retardation | 11/33 (33%)e | 19/218 (9%) | 45/85 (53%) | 17/33 (52%) | Low |
| Hypomelanosis of Ito | 2/33 (6%) | 0/229 (0%) | 2/85 (2%) | NAf | NA |
| Syndactyly | 2/21 (10%) | NA | 13/85 (15%) | NA | NA |
| Kabuki-like facial features | 0/33 (0%) | 0/229 (0%) | 10/64 (15%) | 6/47 (13%) | 0/11 (0%) |
| Short stature | 10/13 (77%) | 50/58 (86%) | 73/75 (98%) | 29/35 (83%) | 7/11 (64%) |
| Edema | 3/29 (14%) | 135/204 (66%) | 14/80 (18%) | 15/34 (44%) | 10/11 (91%) |
- a This population includes 33 subjects from the clinic of V.P.S., but excludes 5 (SA-114, SA-101, SA-111, SA-113, SA-107, ascertained at other clinics) of the 21 subjects listed in Table I, in order to achieve a more unbiased ascertainment.
- b See text for references.
- c Fifteen of these cases were reported within other series and are included in our literature review.
- d 45,X karyotype with no evidence of mosaicism.
- e Number of cases with feature over number of informative cases, with percentage in parentheses.
- f Not reported.
We also compared our clinic population to cases reported in the literature (Table II). For this comparison, we reviewed findings for 85 cases reported in the medical literature from 1980–2002 [Dallapiccola et al., 1980; Berkovitz et al., 1983; Kushnick et al., 1987; Koch et al., 1990; Lin et al., 1990; Guttenbach et al., 1991; Johnson et al., 1991; Grompe et al., 1992; Lindgren et al., 1992; Tharapel et al., 1992; Van Dyke et al., 1992; Dennis et al., 1993; Cole et al., 1994; Collins et al., 1994; Wolff et al., 1994; Cantu et al., 1995; Blumenthal and Allanson, 1997; El Abd et al., 1997; McGinniss et al., 1997; Uehara et al., 1997; Yorifuji et al., 1998; El Abd et al., 1999; Dennis et al., 2000; Kuntsi et al., 2000; Migeon et al., 2000; Turner et al., 2000; Stankiewicz et al., 2001; Tomkins et al., 2002]. We have presented separately the data from a large study of 47 cases in Table II because 15 of the cases in this study were reported earlier and included in our literature review [Dennis et al., 2000; Kuntsi et al., 2000]. Overall, our clinic population appears to be less severely affected, compared to previously reported cases, with lower incidences of mental retardation, Kabuki-like features, and short stature (Table II).
Cytogenetic Analyses
G-banding studies on lymphocyte cultures revealed mosaicism for a 45,X cell line and a 46,X,r(X) cell line in all 21 individuals included in this study. The proportion of cells with a r(X) chromosome varied from 1% to 72% (Table III). The size of the r(X) was evaluated in comparison to the size of chromosome 21. The r(X) was significantly smaller (about 0.25–0.33 the size of chromosome 21) in those individuals with mental retardation (except for SA111), as compared to those with normal intelligence (about 0.33 to two times the size of chromosome 21) (Table III). Fluorescence in situ hybridization (FISH) using a painting probe for the human X chromosome identified the ring present in all 21 cases as derived from X material, with no apparent inclusion of material from other chromosomes (Fig. 1a,b; Table III).
| SAa | MRb | % cells with ringc | Size of ringd | X painte | Distal boundf | XIST FISHg | XIST RT-PCRh | Replicationi | AR FISHj | AR methylk |
|---|---|---|---|---|---|---|---|---|---|---|
| 114 | + | 72 | 0.33 | + | − | − | − | Early | + | − |
| 103 | + | 43 | 0.25 | + | + | + | NDl | Early/late | ND | ND |
| 101 | + | 40 | 0.5 | + | + | + | ND | Early | ND | ND |
| 96 | + | 15 | 0.33 | + | + | + | + | Early | ND | + |
| 106 | + | 15 | 0.33 | + | + | + | + | Early | + | + |
| 121 | + | 32 | 0.33 | + | + | + | ND | ND | + | + |
| 111 | + | 56 | 1 | + | + | + | + | Early | + | ND |
| 110 | + | 35 | 0.25 | + | + | + | ND | ND | + | + |
| 122 | + | 1 | 0.25 | + | + | + | − | Late | + | + |
| 127 | − | 62 | 0.5 | + | ND | + | ND | Late | ND | ND |
| 100 | − | 38 | 3 | + | ND | + | + | Late | + | + |
| 102 | − | 15 | 0.5 | + | + | + | + | ND | + | + |
| 109 | − | 16 | 0.33 | + | + | + | ND | Early | ND | ND |
| 104 | − | 4 | 3 | + | ND | + | ND | Late | ND | ND |
| 105 | − | 11 | 1–2 | + | + | + | + | ND | ND | ND |
| 112 | − | 4 | 2 | + | + | + | ND | Late | ND | ND |
| 113 | − | 19 | 2 | + | + | + | ND | Early | ND | ND |
| 124 | − | 32 | 1 | + | ND | + | + | Late | + | + |
| 123 | − | 28 | 1–2 | + | + | + | + | Late | + | + |
| 126 | − | 44 | 2 | + | ND | + | + | Late | + | + |
| 107 | − | 55 | 1 | + | + | + | ND | Late | ND | ND |
- a Subject identification number.
- b Mental retardation; +, has mental retardation; −, did not have mental retardation.
- c % of 46,X,r(X) cells, all other cells being 45,X.
- d Approximate size of the ring in relation to the size of chromosome 21.
- e FISH using wcpX; +, r(X) was positive.
- f FISH using cIC61; +, r(X) was positive; −, r(X) was negative.
- g FISH using XIST; +, r(X) was positive; −, r(X) was negative.
- h RT-PCR for XIST: +, expression; −, lack of expression.
- i Replication of the r(X) was early or late; early/late indicates a variable replication pattern between cells.
- j FISH using AR; +, r(X) was positive.
- k AR methylation status on the r(X): +, AR was methylated; −, AR was unmethylated.
- l ND, not determined or non-informative.
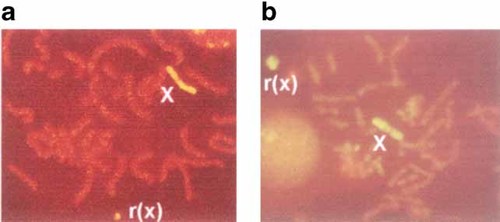
Cytogenetic analysis (a, b), examples of FISH analyses using wcpX on SA96 and SA112, respectively, showing positive hybridization to the r(X). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
XIST Analyses
FISH with a probe that recognizes XIST was completed on all 21 subjects and showed that 20 of these had positive signals on their r(X), indicating that the majority of cases retained XIST (Fig. 2a; Table III). Subject SA114 was the only one who showed absence of XIST FISH signals on her r(X), while signals were present on her normal X chromosome in all cells examined as expected (Fig. 2b; Table III). All other subjects, including eight other individuals with mental retardation (SA103, SA101. SA96, SA106, SA121, SA111, SA110, and SA122) had XIST present on both their r(X) and normal X chromosome (Table III). A cosmid probe cIC61 that recognizes a region of the X chromosome 200 kb distal to XIST was also found to be positive in 15 of the 16 cases examined, with the exception being SA114 who showed deletion of this region (Fig. 2c; Table III). The presence of the region recognized by cIC61 on most r(X) indicated that the breakpoint in the r(X) of most cases was at least 200 kb distal to XIST.
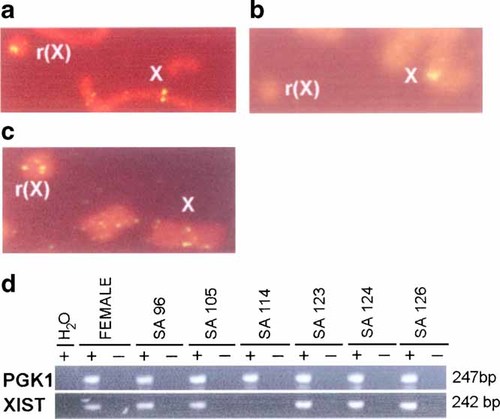
XIST analyses (a), example of FISH using an XIST probe on SA107, showing signals on both the r(X) and the normal X; (b) example of FISH using an XIST probe on SA114, showing a signal on the normal X but not on the r(X); (c) example of FISH using cIC61 on SA123, showing signals on both the r(X) and the normal X; (d) RT-PCR of XIST and a control gene PGK1 on RNA samples from SA96, SA105, SA114, SA123, SA124, and SA126. A control female sample is included. XIST was amplified from all cases, except SA114. Reactions were done with and without RT. www.interscience.wiley.com.]
RT-PCR analysis was done to evaluate XIST expression. RNA was available in eight cases. All were found to have apparently normal XIST expression, except for SA114, as expected from absence of the XIST locus on her r(X) and one other case (SA122) who had a r(X) in only 1% of her cells (Fig. 2d; Table III). In this latter case, the expression of XIST may have been too low for detection, rather than absent.
X Inactivation Analyses
Replication analyses were done using bromodeoxyuridine (BrdU) labeling. We found that in all of our cases, the normal X chromosome was early replicating and thus presumably active, both in cells with and without the r(X), indicating that there was no evidence of random X inactivation in these cases (Fig. 3a,b). An apparently early replicating r(X) was identified in five of nine individuals with mental retardation; one additional case (SA-103) had a ring with both early and late patterns and one case (SA-122) had a late replicating ring (Fig. 3a; Table III). In contrast, most subjects with normal intelligence had a late replicating r(X) (Fig. 3b; Table III). Only 2 (SA-109, SA-113) of 12 cases with normal intelligence had evidence for early replication of the ring and in both of them the replication pattern was difficult to determine.
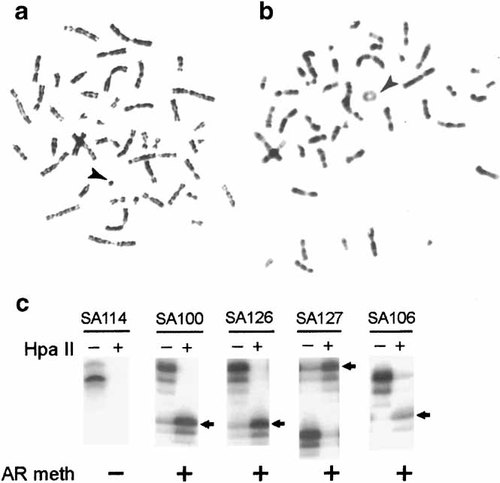
X inactivation analyses (a), examples of replication patterns in SA101 [early replicating r(X) is dark] and SA100 [late replicating r(X) is pale]; (b) methylation of AR in SA114, SA100, SA126, SA 127, and SA106 as determined by digests with a methylation sensitive enzyme followed by PCR using primers to differentiate alleles. DNA digested with RsaI (lanes marked −) or with RsaI and HpaII (lanes marked +) was PCR amplified using primers for HUMARA. Only methylated alleles on inactive X chromosome are amplified following HpaII digestion. In all cases, except SA114, one allele was uncut after HpaII digest (arrow), indicating methylation of AR on the r(X).
By FISH analysis, the AR gene was present on the r(X) of all cases available for this analysis, including SA114 who did not have XIST on her ring (Table III). Because the AR gene is located between XIST and the centromere on the long arm of the X chromosome, its presence on the r(X) indicated long arm breakpoints distal to XIST in all cases examined, except for SA114. In this latter case, the breakpoint must be distal to AR and proximal to XIST, unless a more complex rearrangement occurred in that ring.
Methylation analyses of polymorphic alleles of AR were done to determine the inactivation status of the gene on the r(X). We have extensively used this assay to determine skewing of X inactivation patterns [Woodward et al., 2000]. Control samples with and without skewed X inactivation patterns were examined along with the r(X) cases. In these controls, skewed X inactivation was evident from the lack of digestion of one allele of AR by enzymes (HpaII and HhaI) sensitive to cytosine methylation of CpG dinucleotides, whereas random X inactivation resulted in equal digestion of both alleles (data not shown). In the r(X) cases, it was already known that the normal X chromosome was always active, as determined by replication analyses (see above). Thus, the question asked was whether AR on the r(X) was unmethylated (presumably active) or methylated (presumably inactivated). Methylation of AR on the ring would be apparent by selective digestion of the allele on the normal X by enzymes sensitive to methylation, whereas the allele on the r(X) would remain undigested. This analysis was complicated by the fact that the r(X) was not present in all cells, resulting in a faint signal from the allele on the ring. Therefore, only cases with sufficient band intensity from the r(X) allele to clearly determine the methylation status of each allele were recorded. By these criteria, the AR gene was clearly unmethylated and presumably active on the r(X) of SA114 (Fig. 3c; Table III). In the other ten subjects that were informative, AR appeared methylated and presumably inactivated on the r(X). In six of eight cases where both AR and replication analyses of the r(X) were available, there was concordance between the two measures of inactivation of the r(X). However, in two cases (SA96 and SA 106), discordance between these two measures was apparent, as shown by methylation of the AR allele on a seemingly early replicating ring (Fig. 3c; Table III). Because of the low proportion of cells with r(X) in these two cases, the allele on the r(X), clearly visible in the HpaII-digested lane, was very faint in the undigested lane and it was not possible to compare the band intensities in the HpaII-digested and undigested lanes. We cannot completely rule out the possibility of a mixture of methylated and unmethylated alleles of AR on the r(X) in these cases.
DISCUSSION
The present series of 21 new cases mosaic for a r(X) chromosome partially confirms existing patterns for such cases, mainly that individuals with a r(X) that fails to inactivate due to lack of XIST have a more severe phenotype, whereas those with a r(X) that expresses XIST show inactivation of the ring and a milder phenotype [Duncan et al., 1993; Migeon et al., 1993; Cole et al., 1994; Migeon et al., 1994; Wolff et al., 1994; Callen et al., 1995; Jani et al., 1995; Guillen et al., 1997; McGinniss et al., 1997; Dennis et al., 2000; Turner et al., 2000; Matsuo et al., 2000a; Tomkins et al., 2002]. However, our series significantly adds to the group of individuals who appear to fall outside this standard pattern of phenotype correlated to the presence/absence of XIST and the X-inactivation status of the r(X) [Dennis et al., 1993, 2000; El Abd et al., 1999; Kuntsi et al., 2000; Matsuo et al., 2000a; Migeon et al., 2000; Turner et al., 2000; Stankiewicz et al., 2001]. Indeed, a significant number of our subjects with mental retardation had a r(X) that contained XIST and those who were informative showed AR methylation, indicative of inactivation of the r(X). However, these same individuals often had an early replicating r(X), suggesting the possibility of incomplete inactivation. In contrast, the group of individuals with normal intelligence all had XIST on their r(X), showed AR methylation when informative, and most showed late replication, indicating that their r(X) was inactivated.
Individuals with 45,X/46,X,r(X) cytogenetic constitution comprise about 6–7% of cases with a diagnosis of Turner syndrome [Sybert, 1995]. It has been noted that there is an increased prevalence of mental retardation and other atypical physical abnormalities in this group, in comparison to 45,X cases (Table II). Other features that distinguish r(X) individuals include a higher incidence of seizures, strabismus, triangular face in childhood, ear infections, pigmentary dysplasia (some related to the presence of hypomelanosis of Ito), shorter stature, and smaller head [Kushnick et al., 1987; Van Dyke et al., 1992; Dennis et al., 1993; Collins et al., 1994]. Dennis et al. [1993] specifically examined the facial features and noted the presence of hypertelorism, broad nose, anteverted nostrils, wide mouth with a thin upper lip, and long palpebral fissures. This constellation of findings has been considered by several authors [Dennis et al., 1993; McGinniss et al., 1997; Turner et al., 2000] to be similar to those found in individuals with Kabuki syndrome [Kawame et al., 1999]. Soft tissue syndactyly has been reported in cases with r(X) [Kushnick et al., 1987; Grompe et al., 1992; Van Dyke et al., 1992; Dennis et al., 1993] and may be relatively specific for this cytogenetic abnormality. Other congenital malformations reported once in single cases in the literature include agenesis of the corpus callosum [El Abd et al., 1997], prune belly sequence [Guillen et al., 1997], open neural tube defect, and diaphragmatic hernia [Nowaczyk et al., 1998].
In comparing the clinical findings of our 33 subjects with r(X) (including 16 of the 21 cases in the present report) who have been followed in a multi-specialty clinic by a single investigator (V.P.S.) to cases reported in the medical literature, our findings of a lower incidence of abnormal phenotypes, including mental retardation, Kabuki-like facial features and short stature, suggest a possible bias of ascertainment for those previously reported cases (Table II). For example, dysmorphic facial features atypical of TS, reported to be common in r(X) cases, were observed only in two of our subjects with MR. Dennis et al. [2000] have argued that individuals with a r(X) show no distinctive physical abnormalities, when compared to 45,X TS individuals. When Kabuki syndrome was becoming more recognized as a distinct dysmorphology syndrome in the 1990s, there was an increased number of cases of r(X) noted to have features similar to those observed in Kabuki cases. Although two of our cases have unusual facial features, we were not struck by the presence of either dysmorphic or Kabuki-like features, and in no instance was the diagnosis of Kabuki syndrome entertained in the initial differential diagnosis of subjects within the clinic population followed by V.P.S. Thus, the incidence of dysmorphic facial features in r(X) individuals appears to be low. One consistent finding in r(X) cases is the presence of soft tissue syndactyly, found in 13/85 (15%) of cases in the medical literature and 2/21 subjects (14%) in our clinic population.
It is of interest to compare the TS features of r(X) cases to those of 45,X cases (Table II). TS features likely result from haploinsufficiency of genes that escape X inactivation [Zinn et al., 1993]. Therefore, the presence of a r(X) that retains critical genes that normally escape X inactivation would be expected to provide protection from some TS features. However, the presence of mosaicism for a 45,X cell line in most r(X) cases complicates this issue and such protection would not be manifested in all cases. Interestingly, the incidence of edema was significantly lower in our 45,X/46,X,r(X) subjects, as compared to 45,X individuals, suggesting that many of our subjects retain a region of the X chromosome that must be present in two copies to prevent edema (Table II). Based on analysis of the breakpoints in a series of r(X) cases, Dennis et al. [2000] have suggested that a locus in Xq13.2 may provide protection from edema if both alleles are present. This region of the X chromosome is located very near the XIST gene that is retained in most of our cases.
The wide range of phenotypic features in individuals with a r(X) could potentially be explained by a number of factors including X inactivation status, size, gene content, and level of mosaicism of the ring [Leppig and Disteche, 2001]. Only one of our subjects with mental retardation and other abnormal phenotypic features showed absence of XIST on her r(X) and absence of XIST expression, consistent with lack of inactivation as determined by early replication and absence of AR methylation. The phenotype of this individual is probably due to functional disomy for genes contained in the r(X). Similar cases with an abnormal phenotype have been previously reported [Migeon et al., 1993, 1994; Wolff et al., 1994; Callen et al., 1995; Jani et al., 1995; Guillen et al., 1997; McGinniss et al., 1997; Dennis et al., 2000; Matsuo et al., 2000a; Turner et al., 2000; Tomkins et al., 2002]. However, some individuals who lack XIST on their r(X) have a surprisingly mild phenotype, which may be due to the small size of such rings or their low incidence [Wolff et al., 1994; Turner et al., 2000]. The gene content of the r(X), which has been determined in some cases, is consistent with a linear arrangement of genes located near the centromere on the normal X chromosome, indicating that these rings formed after two breaks, one in the long arm and one in the short arm of the X chromosome [Wolff et al., 1994; Jani et al., 1995; Dennis et al., 2000; Matsuo et al., 2000a; Turner et al., 2000]. However, ring chromosomes can result from multiple breaks and be quite complex [Wong et al., 1989; McGinniss et al., 1992; Fang et al., 1995] (C.M. Disteche, unpublished results). Thus, the gene content of rings of similar size might be variable, which would modify the severity of the phenotype. Furthermore, individuals with low level mosaicism for a small r(X) may be relatively spared the abnormal phenotypic sequelae. In the one severely affected individual reported here, the r(X) lacking XIST was present in a high proportion of cells, at least in blood lymphocytes. An unusual pathway that could ameliorate the phenotype of individuals with a r(X) lacking XIST was proposed by Migeon et al. [1996] who described a r(X) that resulted in XIST deletion but was probably formed after X inactivation had taken place. Since XIST does not appear to be necessary for maintenance of X inactivation [Brown and Willard, 1994], this r(X) remained in an inactive state, thus resulting in a mild phenotype.
The etiology of mental retardation and congenital malformations for the eight individuals described with XIST on their r(X) remains uncertain. Due to the limited amount of sample available, we were not able to determine whether XIST was expressed in all cases; thus some of the rings may contain a silent or non-functional XIST, which could lead to non-inactivation of the r(X), as has been described in some cases [Migeon et al., 1993, 1994; Jani et al., 1995; Yorifuji et al., 1998; Dennis et al., 2000; Tomkins et al., 2002]. However, even in the presence of XIST expression, cases with abnormal phenotypes have been described [Migeon et al., 2000; Stankiewicz et al., 2001]. In two of these cases, a second ring X chromosome that lacked XIST was found, which could explain the abnormal phenotype [Migeon et al., 2000]. We found no evidence for a second ring in any of our cases. The similarity of abnormal phenotypes between r(X) individuals who lack XIST and/or XIST expression and r(X) individuals who express XIST suggest that, in these latter cases, X inactivation may be incomplete, despite XIST expression. Additional factors located in cis on the X chromosome and required for X inactivation could be missing in rings that fail to undergo X inactivation. Our subjects' r(X) contained both the distal boundary of the X-inactivation center [Leppig et al., 1993] and the AR gene located on the other side of XIST, indicating that the region around XIST was intact. One possibility is that a ring may fail to undergo chromatin conformation changes associated with normal X inactivation. Interestingly, our series shows that individuals with mental retardation who have an XIST-bearing r(X) tend to have a small ring chromosome, typically smaller in size than chromosome 21, while those who have normal intelligence have a larger r(X). Thus, a small r(X) may be unable to undergo structural changes associated with formation of the Barr body. On the other hand, our observations may simply result from the fact that individuals with larger r(X) that would fail to become inactivated would be unlikely to survive.
Determination of the X-inactivation status of the r(X) in our cases was made using two indirect methods, replication analysis and AR methylation analysis, whenever possible. The majority of individuals with mental retardation had an early replication pattern, whereas those with normal intelligence had predominantly a late replication pattern. In contrast, our study of the AR gene indicated methylation of one allele, even in cases with an apparently early replicating r(X). This apparent contradiction may indicate dissociation between methylation and late replication in these abnormal chromosomes. Alternatively, the AR methylation or the replication studies could be inaccurate. Replication analysis based on determination of staining properties is especially difficult in small rings (which predominate in the subjects with mental retardation). Interestingly, late replication is not invariably correlated with gene expression in cases of X/autosome translocations [Sharp et al., 2001]. Dissociation between the methylation status of X-linked genes and their replication status has been reported in ICF (immunodeficiency, centromeric instability, and facial abnormalities) syndrome, which is caused by mutations in the DNA methyltransferase DNMT3B [Hansen et al., 2000]. In these individuals, hypomethylation can be associated with late replication and gene silencing. Whether the reciprocal situation of hypermethylation together with early replication and gene expression that can occur is not known for any X-linked gene. Expression analyses of genes contained in the rings, which has been done in a few cases that lacked XIST [Migeon et al., 1993; Migeon et al., 1994], would help resolve the issue of incomplete inactivation in cases where XIST is present.
The prevalence of abnormal phenotypes in individuals with r(X) that contain XIST may also result from events in early embryonic development. The presence of XIST on the r(X) implies that the ring can presumably be counted, resulting in random X inactivation. Therefore, a population of cells with the intact X chromosome inactivated may have been present in early embryo. Such cells would have been nullisomic for a significant portion of the X chromosome. Depending on the rate at which cell selection would eliminate these cells [Gartler and Sparkes, 1963], abnormalities in embryonic development may have ensued. In the case of larger r(X), where cell selection in favor of the normal X being active is also observed, the imbalance would be less deleterious in early embryonic cells with inactivation of the normal X. Indeed, individuals with larger r(X) tend to have a TS phenotype without mental retardation. In an exceptional case with a large r(X) random inactivation persisted in the adult, causing an abnormal phenotype [Matsuo et al., 2000b]. In turn, an abnormal phenotype can also arise from skewing of inactivation of a r(X), by leading to expression of a deleterious allele, as exemplified by a case of Rett syndrome with a mutation of the MECP2 (methyl CpG binding protein 2) gene on the normal X chromosome [Rosenberg et al., 2001].
In conclusion, based on the present study and other studies of individuals with r(X), the phenotype can only be partially predicted based on the size and frequency of the r(X), the presence and expression of XIST, the replication timing, and AR methylation status. Clearly, most individuals with a r(X) have at least some features of TS. Those with the tiniest rings that have a centromere and little or no euchromatic material and are missing XIST usually have no additional abnormal phenotypes. Individuals with high frequency of r(X) chromosomes that contain a centromere and euchromatin, but lack XIST expression are at greatest risk for having a severe phenotype, including mental retardation and congenital malformations. Cases with small r(X) chromosomes that retain XIST are the most difficult to predict. In these individuals, inactivation of the r(X) may not be complete or there may be deleterious effects of random X inactivation in early development. The majority of cases with medium to large size r(X) that are subject to X inactivation often have normal intelligence. Finally, it should be noted that the risk for individuals with a r(X) to have mental retardation may be overemphasized due to bias of ascertainment.
Acknowledgements
This work was supported by the National Institutes of Health, grants P30 HD28834 to K.A.L and GM46883 to C.M.D. The authors thank Dr. Esther White, Pat Lingenfelter, John Wolff, and Wayne Guest for their assistance. They are further grateful to subjects and their families for contributing to this study.




