Autosomal dominant inheritance of infantile myofibromatosis
Abstract
We present three families with infantile myofibromatosis (IM; OMIM no. 228550) inherited in an autosomal dominant (AD) manner. These three pedigrees prompted re-assessment of pedigrees available within the genetic, oncologic, surgical, and pathologic literature, which suggest autosomal recessive (AR) inheritance. All familial IM may be interpreted as AD or, alternatively, there may be genetic heterogeneity for IM. As most nodules tend to regress spontaneously, familial history may be difficult to obtain and/or confirm. Clinical diagnosis and establishment of inheritance pattern can be important for prognosis and the recognition that other family members may be affected. © 2004 Wiley-Liss, Inc.
INTRODUCTION
Infantile myofibromatosis (IM) is the most common type of fibromatosis, or spindle cell tumor, in infancy and childhood. IM tumors are most frequently present at birth or develop in early infancy, and present less often during adulthood. These tumors were previously diagnosed as hamartomas, mesenchymal hamartomatosis, multiple vascular leiomyomas of the newborn, or hemangiopericytomas. Presently, amongst soft tissue pathologists, a diagnosis of hemangiopericytoma is considered synonymous with IM [Dictor et al., 1992]. Stout [1954] first described this entity based on histology and suggested the term “congenital generalized fibromatosis.” Kauffman and Stout [1965] and others [Enzinger, 1965] further sub-categorized IM into solitary, multiple, and generalized forms based on the extent of involvement. In a review of 61 pathologic specimens, Chung and Enzinger [1981] recommended the term IM, as the cells have features of both differentiated fibroblasts and smooth muscle cells (myofibroblasts). IM generally has a zonal architectural pattern with more primitive appearing cells in the center of lesions and spindle cells with a more myoid appearance at the periphery. Necrosis and calcification are also characteristic, and are often noted in the central zone. Myofibroblasts, which stain for actin and vimentin but not desmin, are also present [Fisher, 1996].
While solitary, multiple, and generalized IM all demonstrate skin and soft tissue involvement, the multiple form also involves bone, and the generalized form also involves viscera [Coffin et al., 1995]. Soft tissue lesions tend to be isolated and regress spontaneously, without need for medical intervention. Individuals with visceral involvement tend to have higher rates of morbidity and mortality, often requiring surgical intervention. While variable, their clinical course can include pulmonary, cardiac, and GI complications, and even intra-cranial involvement [Wiswell et al., 1988; Coffin et al., 1995; Kaplan et al., 2002].
Presence of consanguinity in a few published pedigrees has led some to interpret the inheritance pattern of IM as autosomal recessive (AR) [Baird and Worth, 1976; Salamah et al., 1988; Narchi, 2001]. However, a few pedigrees reported are autosomal dominant (AD) [Bartlett et al., 1960; Pfluger et al., 1976; Jennings et al., 1984]. We present three new families with clinical and pathologic confirmation of IM in at least two generations and re-review the 12 pedigrees that have been previously published. We suggest that there is either genetic heterogeneity or that all pedigrees are consistent with AD inheritance with variable penetrance and expressivity.
FAMILY HISTORIES
Family I
The proband (Fig. 2; III-10) presented at birth with a 3 × 3 cm2 left upper quadrant abdominal wall mass in the superficial soft tissue. At 3 weeks of age, an additional mass was noted behind his left knee, while at 6 weeks of age the abdominal wall mass regressed. Familial history was notable for a brother (Fig. 1A; III-9) and father (II-6) who were diagnosed with biopsy proven IM. Additional family members also carry the diagnosis (I-1, I-3, II-2, II-3, III-3, III-4, and IV-1), either through pathologic confirmation or clinical history. The clinical course of all affected individuals was consistent with solitary IM, and no family member reported adverse complications.
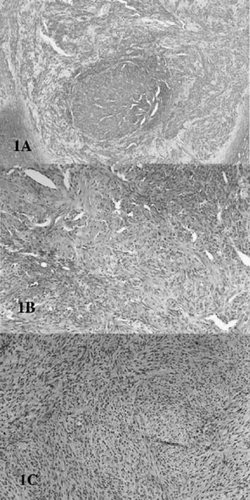
Hematoxylin and eosin staining of infantile myofibromatosis (IM) biopsies. A: Family I (III-9), showing zonal pattern of spindle shaped cells with central necrosis and calcification. The lesion was a subcutaneous scalp mass obtained at 4 months of age, and the diagnosis of IM was confirmed by outside consultation (Dr. C. Coffin, U. of Utah). B: Family II (IV-6), shoulder lesion obtained at 3 months of age, but present since birth. The sample demonstrates prominent vascularity. C: Family II (III-5), temporal lesion, biopsed at age 28 years. Diagnoses initially considered included fibroblastic meningioma, Schwanoma-neurilemmona, and IM. The patient has generalized IM confirmed by multiple other biopsies of the deltoid, axilla, and shoulders. Note the architectural similarity of (B) and (C) despite their different origins.
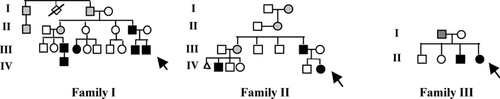
Case history pedigrees of families I, II, and III.
Family II
The proband was initially evaluated at 3 years of age due to a persistent “scalp mass,” which had presented during infancy. The scalp mass and an additional soft tissue mass on her shoulder were biopsed and diagnosed as IM (Fig. 1B). At her present age of 16 years, she has developed a total of six soft tissue lesions without complication (Fig. 2; IV-6).
Both her father (Fig. 1C; III-5) and paternal first cousin (IV-2) have been diagnosed with biopsy proven IM, but have had a much more complicated clinical course. Her father developed both soft tissue and visceral lesions throughout his life, including lesions in his meninges, kidney, and vocal cords, requiring tracheotomy placement. At 9 months of age the proband's first cousin developed an intususseption. Pathology obtained during the surgical repair was consistent with multiple myofibromata, which presumably served as a lead point to the intususseption. Subsequent biopsies of other soft tissue lesions were also consistent with IM.
Other family members (I-2, II-2, and III-2) have reported soft tissue “lumps and bumps,” which were surgically removed. However, further information regarding clinical courses and pathologic diagnoses cannot be obtained.
Family III
The proband (Fig. 2; II-4) presented at birth with a large (7 × 6 × 3 cm3) pedunculated mass attached to her right parieto-occipital scalp by a 2.5 × 2.5 cm2 stalk. Cranial imaging demonstrated this to be a soft tissue mass with no underlying skull defect. The lesion was surgically removed on the second day of life and pathologic findings revealed solitary IM. She is doing well at her present age of 6 months. Her otherwise healthy 2-year-old brother (II-3) had previously undergone surgery for a small thigh mass at 10 weeks of age. This lesion had developed at 8 weeks of age, measured 1.5 × 1.3 × 1 cm3, and was also found to be a myofibroma.
Her father (I-1) reported having subcutaneous nodules on each thigh that have regressed.
LITERATURE
The pedigrees in Figure 3A suggest AD inheritance. Bartlett et al. [1960] was the first familial report of IM. Two sibling pairs (III-2 through III-5), related as first cousins, were diagnosed with either solitary, multiple, or generalized IM. By history, one parent (II-3) may have also carried a diagnosis of IM. In Pfluger et al. [1976], three generations of a family with both clinical and pathologic confirmation of solitary IM were reported. By history, other family members may have been affected. Jennings et al. [1984] reported a newborn infant (III-1) with multiple IM and her father (II-2), who had a history of solitary IM. The father developed an uncomplicated sublingual lesion in his 20's, changing his classification to generalized IM.
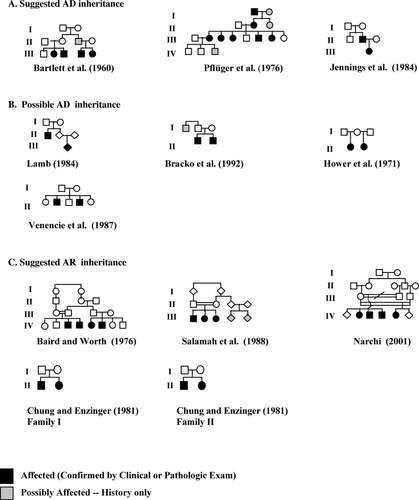
Literature pedigrees suggested as: (A) Autosomal dominant (AD) inheritance, (B) possible AD inheritance, or (C) autosomal recessive (AR) inheritance.
The pedigrees in Figure 3B can be interpreted as AD inheritance. As an addendum to the report by Jennings et al. [1984], Lamb reported a newborn infant (III-1) whose uncle (II-1) was also diagnosed 30 years prior with IM. The classification of either patient was not reported. Given the uncle's confirmed diagnosis, one could postulate that his sibling, the parent (II-2) of the proband (III-1), had an undiagnosed nodule, which would be consistent with AD inheritance. Bracko et al. [1992] reported two brothers (II-1 and II-2) with pathologically confirmed multiple IM and a paternal uncle with a history suggestive of solitary IM. In this family, one could speculate that the father (I-2) might have had an undiagnosed IM lesion, making the pedigree consistent with AD inheritance. Hower et al. [1971] reported two half sisters with either multiple (II-1) or generalized (II-2) IM. If the girls' mother (I-2) had undiagnosed IM secondary to nodular regression, AD inheritance would be clear. Venencie et al. [1987] reported two brothers, the oldest (II-2) with multiple IM, and the other (II-4) with probable solitary IM. Three siblings and both parents were without known lesions. Either parent could have had an undiagnosed nodule, which would indicate AD transmission.
The pedigrees in Figure 3C were interpreted previously as having AR inheritance due to consanguinity or a single generation of affected siblings. However, they can also be interpreted as AD inheritance with incomplete penetrance. In the report by Baird and Worth [1976], four affected first cousins (two sibling pairs; IV-3 through IV-6) with either solitary or multiple IM were presented. The parents of two siblings were second cousins (III-1 and III-2). The parents of the other two siblings (III-3 and III-4) were not known to be consanguineous. If other family members, such as I-1 and I-2 had unrecognized or undiagnosed nodules, inheritance could easily be transmitted to generation IV by AD inheritance. Alternatively, II-3 may have been solely responsible for transmission, making the history of consanguinity inconsequential.
Three siblings with solitary IM (III-1 through III-3) reported by Salamah et al. [1988] (III-1 through III-3) were born to parents who were first cousins. Clinical histories of the first cousins of these children (III-4 and III-5) were suggestive of IM, though pathology or clinical exam did not confirm this. Their parents (II-3 and II-4) were not known to be related. An AD pattern may be recognized by transmission through both grandparents (I-1 and I-2) or, alternatively, through I-2 alone.
Three siblings (IV-2 through IV-4) reported by Narchi [2001] born to consanguineous parents were reported with either solitary or generalized IM. Their mother (III-1) re-married the half-brother (III-3) of her first husband (III-2), who was also related. One of their children (IV-5) was also diagnosed with IM. No other family members were known to carry the diagnosis. Consanguinity complicates this pedigree. However, to simplify the picture, if the mother (III-1) of all the children were to have had an undiagnosed nodule, transmission could easily be consistent with AD inheritance. Alternatively, each of the half brothers could have been affected, passing IM as a dominant trait to each of the children.
Chung and Enzinger [1981] reported two families. In Family I, a brother (II-1) and sister (II-2) demonstrated nodules in both soft tissue and bone. The brother's lesions were all over his body, while the sister's localized to the right lower extremity. The medical history of other family members was not reported. AD transmission could occur through either parent unknowingly having a history of a regressed or undiagnosed nodule. In Family II, a brother (II-1) and sister (II-2) with both soft tissue and bone lesions were reported. The sister presented at birth, while the brother initially presented at the age of 8 years. No other family members were reported and AD transmission could occur as in Family I.
DISCUSSION
IM is the most common fibromatosis of infancy. However, the natural history of spontaneous regression of most IM nodules makes a familial history difficult to confirm, or even to obtain, as members may not divulge histories of “lumps and bumps,” that resolved without surgical intervention. All of the families presented may have other members who experienced spontaneous regression of IM nodules without report, both with suspected AR and AD inheritance. Early generational history may also be complicated because there was only recent standardization of pathologic and clinical nomenclature.
Myofibromas are benign; they have no metastatic potential. Morbidity and mortality for both children and adults is associated directly with visceral involvement [Chung and Enzinger, 1981; Coffin et al., 1995]. The initial case histories of generalized IM described were often derived from autopsy, with GI or pulmonary compromise [Stout, 1965; Chung and Enzinger, 1981; Wiswell et al., 1985]. The proband's cousin (IV-2) in Family II (Fig. 2) developed an intususseption from bowel myofibromata and had timely surgical intervention. Chung and Enzinger [1981] also reported this association. Within the same family, the proband's father has experienced progressive and persistent IM in multiple viscera, with the most serious complication thus far being vocal cord paralysis.
The 15 families compiled here allow for comparison of intra- and inter-familial variability of IM subtypes. Within a family, members may be diagnosed with multiple forms of IM (See Table I). Members of Family II manifested different forms of IM (solitary and generalized), as did families reported by Bartlett et al. [1960], Hower et al. [1971], Baird and Worth [1976], Jennings et al. [1984], and Narchi [2001]. In contrast, all members of Family III and I (Fig. 2) are classified as solitary IM.
| Publication | Solitary/multiple/generalized |
|---|---|
|
Bartlett et al. [1960] |
Solitary/multiple/generalized |
|
Pfluger et al. [1976] |
Solitary |
|
Jennings et al. [1984] |
Solitary/generalized |
|
Lamb [1984; addendum noted to Jennings et al., 1984] |
Unknown |
|
Bracko et al. [1992] |
Solitary/multiple |
|
Hower et al. [1971] |
Multiple/generalized |
|
Venencie et al. [1987] |
Solitary |
|
Baird and Worth [1976] |
Solitary/multiple |
|
Salamah et al. [1988] |
Solitary |
|
Narchi [2001] |
Solitary/generalized |
|
Chung and Enzinger [1981]—Family I |
Multiple |
|
Chung and Enzinger [1981]—Family II |
Multiple |
| New pedigrees | |
| Family I | Solitary |
| Family II | Solitary/generalized |
| Family III | Solitary |
AD inheritance may be a consistent inheritance pattern with all of the 15 pedigrees presented. It is quite possible that either incomplete penetrance or lack of identification due to the natural history of tumor regression may have confounded the available familial histories. Alternatively, there may be heterogeneity of inheritance with AD and AR inheritance. Identification of molecular etiology will require additional large pedigrees to help confirm either a single inheritance pattern, or two different inheritance patterns.
Presently, a clear etiology for tumor growth has not been identified. Stenman et al. [1999] reported an interstitial deletion of 6q (del(6)q12q15) detected by both cytogenetic and molecular studies in the tumor of a 6-month-old infant. Further studies were not done to distinguish whether the 6q deletion was limited to the tumor or present in the germline. The report of a child with Turner syndrome treated with interferon alpha is the only other report in the literature of a patient with a cytogenetic abnormality, either constitutive, or somatic [Sava'an et al., 1998].
The mechanism of tumor regression in IM is also not well understood. Two reports have suggested that apoptosis may play an integral role: Fukasawa et al. [1994] and Iijma et al. [1999]. The study by Fukasawa et al. [1994] suggested that analysis of TdT mediated uptake of biotinylated-dUTP in areas of necrosis was consistent with apoptosis. However, other reports have suggested that the growth of these nodules may correspond to angiogenic stimulation [Leaute-Labreze et al., 2001], and regression may be related to the overgrowth of the vascular supply. They propose that the central areas of necrosis common in these samples are a result of this mechanism, and not necessarily apoptosis. Sava'an et al. [1998] treated a patient with interferon-alpha to inhibit angiogenic stimulation. In general, there are too few studies available to discriminate between apoptosis and necrosis-mediated regression. Some groups have attempted chemotherapy for individuals with severe and progressive generalized disease [Fleishhack et al., 1994; Coffin et al., 1995; Day et al., 2002]. However, presently there is no consensus between chemotherapy, interferon therapy or even if medical therapy is indicated in the severe cases of generalized IM. These current questions regarding appropriate interventions for generalized IM most likely reflect the fact that we still do not understand the variability of penetrance, variability of expressivity, and mechanisms of both growth and regression for IM.
Acknowledgements
We appreciate Dr. Brian Rubin (University of Washington) for his helpful suggestions and commentary, and Dr. Jennifer Morrissette and Ms. Livija Medne for critical review of the article.




