Recurrence of achondrogenesis type II within the same family: Evidence for germline mosaicism
Abstract
Achondrogenesis type II is a lethal skeletal dysplasia caused by new dominant mutations within the type II collagen gene (COL2A1). Here we report on two pregnancies of a healthy, nonconsanguineous young couple. In the first pregnancy, severe micromelia and generalized edema were noted on ultrasound at 21 weeks' gestation. Clinical, radiological, and histological evaluation of the fetus after termination of the pregnancy showed typical findings of achondrogenesis type II. In the second pregnancy, fetal hygroma was noted at 11 weeks' gestation. Similar clinical, radiographic, and histologic findings were observed in the second fetus, suggesting the recurrence of achondrogenesis II within this family. Molecular analysis of genomic DNA extracted from amniotic cells of the second fetus revealed heterozygosity for a 1340G > A missense mutation (G316D) in the COL2A1 gene. This mutation was not found in the parents. Although, we could not evaluate the presence of this mutation in the first fetus, we strongly believe that our data are in favor of germline mosaicism as the most likely explanation for the recurrence of type II achondrogenesis in both sibs. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Achondrogenesis type II is a lethal skeletal dysplasia, characterized (i) clinically by severe micromelic dwarfism with small thorax and prominent abdomen, enlarged cranium and cleft palate and (ii) radiologically by incomplete or absent ossification of the vertebral bodies in association with shortening of the tubular bones and hypoplastic pelvis [Chen et al., 1981; Mortier et al., 2000]. New dominant mutations within COL2A1 have been identified in cases with achondrogenesis type II [Mortier et al., 1995; Mortier et al., 2000]. Dominant mutations in the COL2A1 gene can also cause hypochondrogenesis (MIM# 200610), spondyloepiphyseal dysplasia (SED) congenita (MIM# 183900), SED Namaqualand type (MIM# 142670), spondyloepimetaphyseal dysplasia Strudwick type (MIM# 184250), Kniest dysplasia (MIM# 156550), spondyloperipheral dysplasia (MIM# 271700), mild SED with precocious osteoarthritis, and Stickler dysplasia type I (MIM# 108300) [Sher et al., 1991; Spranger et al., 1994a; Bonaventure et al., 1995; Zabel et al., 1996]. Here we report on the first observation of recurrence of achondrogenesis type II in two fetuses from the same parents.
CLINICAL REPORT
The two cases are the product of a respectively second and third pregnancy of a healthy, nonconsanguineous couple. The family history is unremarkable. The father is of German and Polish origin, and the mother is of French origin. The first pregnancy was uneventful and a healthy girl was born at term. Both parents were aged 30 years at time of the second pregnancy. Severe micromelia and generalized edema were noted on ultrasound at 21 weeks' gestation (WG) and a lethal chrondrodysplasia, such as thanatophoric dysplasia, was suspected. Cytogenetic studies performed on blood cord lymphocytes revealed a normal male karyotype: 46,XY. After termination of the pregnancy at 24 WG, a male fetus with severe micromelic dwarfism was born. Measurements were 20 cm for vertex-heel length (≪3rd centile), 406 g for weight (≪3rd centile), and 21 cm for occipitofrontal circumference (OFC) (3rd centile). Clinical evaluation revealed generalized edema with short trunk and micromelia (Fig. 1). The thorax was small and narrow and the abdomen prominent. The head was relatively large with flat face, hypertelorism, short nose, anteverted nostrils, and low-set, posteriorly rotated ears. The extremities were very short with clinodactyly of the fifth fingers and bilateral single palmar crease. Radiographs showed typical features of achondrogenesis type II, including very short and broad tubular bones with metaphyseal cupping; abnormal pelvis with short iliac wings and absent ossification of the ischiadic bones; absent ossification of the cervical and sacral vertebral bodies (Fig. 2a,b). Histologic evaluation showed hypercellularity, markedly enlarged chondrocytes, hypervascular matrix in the resting cartilage, and disorganized growth plate, both compatible with achondrogenesis type II (Fig. 3).
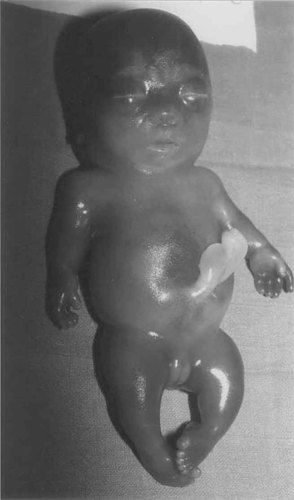
Clinical picture of fetus 2 at 20 weeks of gestation showing the generalized edema, large head, small thorax, protuberant abdomen, and short limbs.
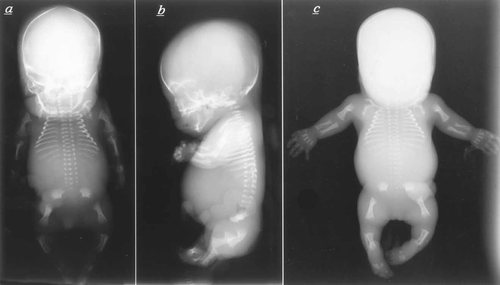
Radiographs of fetus 1 at 24 WG (a,b) and fetus 2 at 20 WG (c). Both fetuses show similar radiographic abnormalities including absent ossification of the vertebral bodies in the cervical and sacral region, hypoplastic vertebral bodies in the remaining spine, pelvis with absent ossification of ischial and pubic bones, flat acetabular roofs, and short and broad tubular bones in the limbs with metaphyseal cupping and translucencies. Evidence of both sagittal and coronal clefting is associated with posterior vertebral body ossification defects.
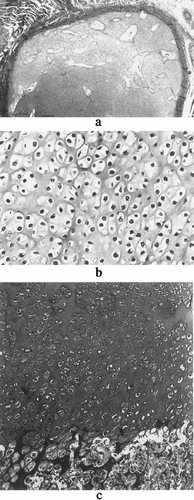
Histologic findings in fetus 1. The resting cartilage is hypervascular (a) and hypercellular with markedly enlarged chondrocytes (b). Growth plate was grossly linear, without recognizable proliferative or hypertrophic zones, giving rise to large trabeculae of mineralized cartilage (c).
One year later, the mother was referred in her third pregnancy at 11 WG because of fetal hygroma. Cytogenetic studies performed on amniocytes were normal (46,XX). Ultrasound survey at 17 WG revealed severe intrauterine growth retardation with micromelia and generalized edema. Termination of the pregnancy was performed at 20 WG. Measurements were 17 cm for vertex-heel length (≪3rd centile), 210 g for weight (≪3rd centile), and 18.5 cm for OFC (3rd centile). Similar clinical, radiographic, and histologic abnormalities as compared to the first fetus were observed in this second fetus, suggesting recurrence of achondrogenesis type II within this family (Figs. 1 and 2).
METHODS AND RESULTS
Genomic DNA (gDNA) of the second fetus and his parents was isolated from cultured amniotic cells and lymphocytes, respectively following standard procedures. PCR amplification, single-strand conformation polymorphism (SSCP) analysis, and direct sequence analysis of abnormal SSCP patterns were performed as previously described [Mortier et al., 2000]. Molecular analysis of gDNA of the second fetus revealed heterozygosity for a 1340G > A transition, predicted to result in a glycine to aspartate substitution (G316D) in the COL2A1 gene (Fig. 4). This mutation was not found in gDNA extracted from the parental blood lymphocytes. Unfortunately, we could not prove the presence of the 1340G > A mutation in the first fetus because of lack of appropriate materials. In addition, the father refused analysis of his sperm cells.
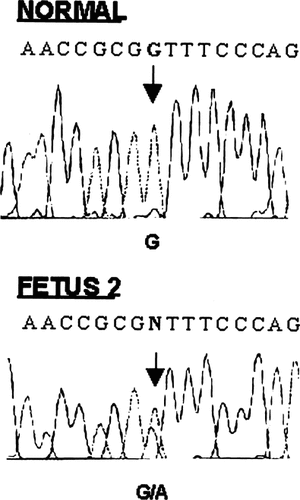
Partial sequence of exon 22 of the COL2A1 gene showing heterozygosity for the 1340G > A mutation in fetus 2.
DISCUSSION
In this study, we report the recurrence of achondrogenesis type II in two fetuses of healthy parents. Heterozygosity for a G316D mutation in COL2A1 was identified in the second fetus. The most likely explanation of this observation is the presence of germline mosaicism in one of the parents. This family is the first illustration of germline mosaicism in achondrogenesis type II, which is relevant for genetic counseling.
Both achondrogenesis type II and hypochondrogenesis represent a phenotypic spectrum at the severe end of the type II collagenopathies. The distinction between the two entities is usually artificial because of considerable overlap between the two disorders [Maroteaux et al., 1983; Borochowitz et al., 1986]. Mainly based on the histology and clinical features we decided to call the condition in this family achondrogenesis type II although the degree of vertebral ossification was more suggestive for hypochondrogenesis.
Germline mosaicism for a dominant mutation in one parent can mimic autosomal recessive inheritance when two or more children are born to apparently normal parents. In the case of germline mosaicism, phenotypically normal individuals may transmit several gametes that are clonal descendants of a single progenitor cell in which a de novo mutation occurred during early embryonic development [Hall, 1988]. In the case of somatic mosaicism, the manifestation of such a mutation in a mosaic parent may range from none or minimal to a severe generalized effect. It could also result in a milder but different phenotype such as the observations of severe Kniest dysplasia in two unrelated children with COL2A1 mutations and mild Stickler syndrome or spondyloepiphyseal dysplasia in their mosaic parents [Spranger et al., 1994b]. Germline/somatic mosaicism has been documented in other autosomal dominant skeletal dysplasias, such as achondroplasia [Fryns et al., 1983], pseudoachondroplasia [Ferguson et al., 1997], and osteogenesis imperfecta [Cohn et al., 1990]. The possibility of germline mosaicism should always be considered in case of an apparently de novo dominant mutation and the family should not be counseled that the recurrence risk is zero. It is difficult to estimate the exact recurrence risk for healthy parents with only one child affected, although this risk is quite low taking into account that achondrogenesis type II/hypochondrogenesis is not that infrequent and there have been no other written reports of recurrence. The empiric risk for osteogenesis imperfecta type II (6%) does not apply here and appears to be unique to osteogenesis imperfecta. Based on general statistical inference, the recurrence risk for a dominant disorder would be less than 5% if only one sib was affected and 20–35% if two or more sibs were affected, depending on the proportion of germ cells carrying the mutation [Bakker et al., 1989].
In the case of a clear radiographic/histologic diagnosis, it is not necessary to perform molecular testing in families with a single affected child. An eventual recurrence of achondrogenesis type II in a subsequent pregnancy can be picked up by ultrasound at the earliest around the 13th–14th week of gestation [Soothill et al., 1993]. Important early ultrasonographic findings include polyhydramnios, nuchal edema, reduced rump length, poor ossification of the vertebral bodies, and shortening of the tubular bones. Yet, the risk of obtaining an early prenatal diagnosis sample would excess the risk of having another affected child.




