UBE3A gene mutations in finnish Angelman syndrome patients detected by conformation sensitive gel electrophoresis†
Katrin Rapakko and Hannaleena Kokkonen contributed equally to the study.
Abstract
Angelman syndrome (AS) is a neurogenetic disorder associated with a loss of maternal gene expression in chromosome region 15q11-q13 due to either maternal deletion, paternal uniparental disomy (UPD), imprinting mutation, or mutation in the UBE3A gene. UBE3A encodes an ubiquitin-protein ligase and shows brain-specific imprinting. We have done conformation sensitive gel electrophoresis (CSGE) mutation analysis of the UBE3A coding region in nine AS patients, who had shown a normal biparental inheritance and methylation pattern of the 15q11-q13. Disease-causing mutations were identified in five of them: three deletions (1930delAG, 3093delAAGA) and two missense mutations (902A → C, 975T → C). Both deletions have also been detected in other AS patients, suggesting these sites may be prone to deletions in the UBE3A gene. All AS cases were sporadic, but a mosaicism for mutation 902A → C was present in a patient's mother. Screening for the UBE3A mutations in the AS patients was found useful both for the confirmation of diagnosis and genetic counseling. CSGE was found to be a sensitive and simple screening method for these mutations. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Angelman syndrome (AS) [MIM 105830] is a neurobehavioral disorder which is characterized by severe mental retardation, ataxic gait, absence of speech, abnormal EEG, seizures, inappropriate laughter and smiling, and dysmorphic facial features [Angelman, 1965; Williams et al., 1995]. The incidence of AS is estimated to be about 1 in 20,000 live births [Clayton-Smith and Pembrey, 1992], and it is caused by a loss of maternal gene expression of chromosome region 15q11-q13. The most common defect is a maternally derived deletion of 15q11-q13 (about 70%) [Knoll et al., 1989]. Paternal uniparental disomy (UPDpat) of chromosome 15 is found approximately in 4% [Malcom et al., 1991], and imprinting defects are responsible for 6% of the cases [Sutcliffe et al., 1994; Buiting et al., 1995]. About 5% of AS patients have been shown to have a mutation in the UBE3A gene [Kishino et al., 1997; Malzac et al., 1997; Matsuura et al., 1997; Fang et al., 1999], while no molecular defect has so far been found in the rest of the patients (15%).
The UBE3A gene has been shown to exhibit tissue-specific imprinting with maternal allele expression in hippocampal neurons and Purkinje cells in mice [Albrecht et al., 1997] and in human brain [Rougeulle et al., 1997; Vu and Hoffman, 1997]. The UBE3A gene encodes E6-AP ubiquitin protein ligase, an enzyme containing two independent, separable functions: coactivation of the nuclear hormone receptor superfamily [Nawaz et al., 1999] and ubiquitin-ligase activity [Huibregtse et al., 1993a]. In the pathogenesis of AS, only a defect in the ubiquitin-proteosome protein degradation pathway seems to result in the AS phenotype [Nawaz et al., 1999]. The UBE3A gene consists of at least 16 exons that span approximately 120 kb, with transcription oriented from telomere to centromere [Kishino and Wagstaff, 1998]. Ten of these exons are protein-coding, while six upstream exons are primarily non-coding, possibly contributing to multiple isoforms of the protein [Yamamoto et al., 1997]. Here, we report mutational analysis of the coding region of the UBE3A gene in nine AS patients in whom deletion, UPD, and imprinting defect had been excluded. We used conformation sensitive gel electrophoresis (CSGE) analysis as a screening method.
MATERIALS AND METHODS
Patients and Control Group
The patient group consisted of nine clinically diagnosed AS patients (see Table I) and their parents. The patients were referred by either clinical geneticists or child neurologists. In these patients deletion of 15q11-q13, paternal UPD of chromosome 15 and imprinting defects had been excluded. All cases were sporadic.
| Patient | AS1 | AS2 | AS3 | AS4 | AS5 | AS6 | AS7 | AS8 | AS9 |
|---|---|---|---|---|---|---|---|---|---|
| Sex | f | f | f | f | m | f | f | f | m |
| Age (years) | 11 | 8 | 3 | 32 | 5 | 2 | 10 | 11 | 3 |
| Consistent features (100%)a | |||||||||
| Developmental delay, functionally severe | + | + | + | + | + | + | + | + | + |
| Absence of speech | + | + | + | + | + | + | + | + | + |
| Ataxia | + | + | + | + | + | + | + | + | + |
| Inappropriate laughter/smiling | + | + | + | + | + | + | + | + | + |
| Frequent features (80%)a | |||||||||
| Microcephaly | + | + | + | + | − | + | + | + | |
| Seizures | + | + | + | + | + | + | + | + | + |
| Abnormal EEG with characteristic pattern | + | + | + | + | + | + | + | ||
| Associated features (20–80%)a | |||||||||
| Flat occiput | + | + | − | + | + | + | |||
| Occipital groove | + | + | + | ||||||
| Protruding tongue | − | + | |||||||
| Tongue thrusting: Suck/swallowing disorders | + | − | − | + | |||||
| Feeding problems during infancy | + | + | + | − | + | + | |||
| Prognathia | + | + | + | + | − | ||||
| Wide mouth, wide-spaced teeth | + | + | + | + | + | + | |||
| Frequent drooling | + | − | + | + | + | + | + | − | |
| Excessive chewing/mouth behaviors | − | + | + | + | |||||
| Strabismus | − | + | + | + | − | − | |||
| Hypopigmentation | − | − | + | − | |||||
| Uplifted, flexed arm position especially during ambulation | + | + | − | + | + | − | |||
| Increased sensitivity to heat | |||||||||
| Sleep disturbance | + | + | − | + | − | + | + | ||
| Attraction to/fascination with water | + | − | + | + | |||||
- +, Feature present; −, feature absent; empty, not recorded.
- a Grouped according to the clinical criteria described by Williams et al. [1995].
As a control group, we studied DNA from 100 unrelated Northern Finnish individuals for the presence of the point mutations detected in AS patients.
CSGE Analysis
Genomic DNA was extracted from peripheral blood. The CSGE analysis included the protein-coding exons except exon 7, which was analyzed by sequencing, using primers described by Kishino and Wagstaff [1998] for single-strand conformation polymorphism (SSCP) analysis (see Table II). The primers were designed to include at least 60 bp both of the 5′- and the 3′-flanking sequences of the target sequence to detect correctly all the mismatches. To generate heteroduplexes, the samples were denatured at 98°C for 5 min, annealed at 68°C for 30 min, and stored at 4°C. PCR products were electrophoresed in a mildly denaturing 10% polyacrylamide gel, stained with ethidium bromide, visualized by an UV transilluminator, and photographed as described by Körkkö et al. [1998]. All the band shifts were verified by repetition of the PCR and CSGE.
| Exon and primer | Sequence | Region | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|---|
| 7* | ||||
| EX1A | GCTAACTGTTTCTCAATTGC | Intron | 55 | 105 |
| EX1B | ATAAGAACCACAGTCTCAAC | Intron | ||
| 8 | ||||
| E21A | GCCTTGATGATATGTTGAGC | Intron | 60 | 365 |
| E21B | AATTCTAGCGCCTTTCTTGT | Exon | ||
| E22A | GCCTGCACGAATGAGTTTTGT | Exon | 62 | 385 |
| E22B | AGTTATTATTCCTGTCCGTTACCA | Intron | ||
| 9 | ||||
| E31A | TGTTTGGCTGTTTTACTTTTAGAA | Intron | 60 | 436 |
| E31B | GGCATCAATATCCACAGACACA | Exon | ||
| E32A | ATGTTCTGCTGCTGCTATGG | Exon | 57 | 424 |
| E32B | TCTCCGAATCTGGTCTGC | Exon | ||
| E33A | ACCTAACGTGGAATGTGACTTGAC | Exon | 65 | 483 |
| E33B | ACTCGAGGACCTTTCTTGTTTCTT | Exon | ||
| E34A | TAGTGGGAGGGGAAGTGGACA | Exon | 64 | 473 |
| E34B | TACCCGGACAAGTGCATCATCTA | Exon/intron | ||
| E35A | CGCATGTACAGTGAACGAAGAA | Exon | 57 | 282 |
| E35B | TGCACAGGAACAACAAAAGTAT | Intron | ||
| 10 | ||||
| E4A | GTTTGCTTTCTGTTTCCATTTAC | Intron | 60 | 392 |
| E4B | ATCCTTCTTTTGCTGCTCTTC | Intron | ||
| 11 | ||||
| E5A | ATTCCTTTGGCTTCATAA | Intron | 50 | 587 |
| E5B | TGGTACTTCGGTCAGATT | Intron | ||
| 12 | ||||
| E6A | AACTATTTGGGGGACTGGAG | Intron | 60 | 592 |
| E6B | ACACCCTGCTTCTTGCTTTAT | Intron | ||
| 13 | ||||
| E7A | GAAATTGTTAAGAAGTAGGTG | Intron | 53 | 394 |
| E7B | ATATGTCTTAGTTATCTGCTA | Intron | ||
| 14 | ||||
| E8A | AGGTGTCTGCAAAAAGTC | Intron | 57 | 332 |
| E8B | TTAGCTCTGAAAAATGGTG | Intron | ||
| 15 | ||||
| E9A | ATAATGAATGCCAAACTGAA | Intron | 53 | 256 |
| E9B | ATATGTATGTGACGAGGAATG | Intron | ||
| 16 | ||||
| E10A | TATTTCCCATGACTTACAG | Intron | 50 | 264 |
| E10B | AAAATTTATCCCTCGTTA | 3′ UTR | ||
DNA Sequencing
PCR products were analyzed by manual sequencing (Cyclist Exo−Pfu DNA Sequencing Kit, Stratagene, La Jolla, California, USA) or by an automated DNA sequencer (LI-COR, Inc., Lincoln, Nebraska, USA). When both primers were inside the exon, the sequence of the mutant allele was compared to the UBE3A sequence and to that of the processed pseudogene UBE3AP2 [Kishino and Wagstaff, 1998], to verify that the mutation affected UBE3A. In the case of suspected mosaicism, two independent instances of sequencing of abnormal fragments of the mother and the child were done.
RESULTS
CSGE analysis detected DNA alterations in six of the nine patients: four in exon 9 and two in exon 16 (Fig. 1, Table III). After sequencing, three of these turned out to be deletions that cause frameshifting and premature chain termination: a 2 bp deletion in exon 9 (1930delAG) in patient AS1 and a 4 bp deletion in exon 16 (3093delAAGA) in patients AS2 and AS3. The parents of the patients had normal CSGE results.
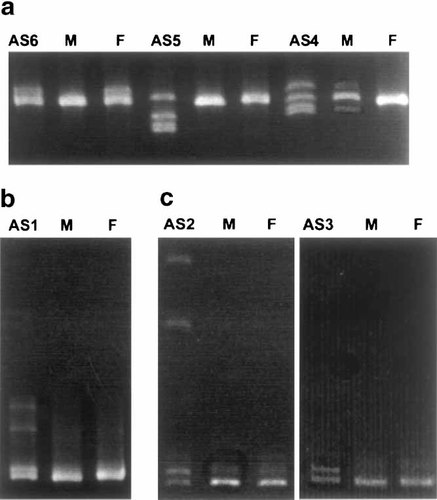
Abnormal bands detected in the coding region of the UBE3A gene by conformation sensitive gel electrophoresis (CSGE). a: Exon 9 (primer pair E31); b: Exon 9 (primer pair E34); c: Exon 16 (primer pair E10). AS1–AS6, patients; M, mother; F, father. The mother of patient AS4 has similar but fainter abnormal bands. Patient AS6 has inherited the abnormal band from her father.
| Patient | Exon | Sequence change* | Type of mutation | Predicted consequence | Other observations |
|---|---|---|---|---|---|
| AS1 | 9 | 1930delAG | Frameshift | Truncated protein | |
| AS2 | 16 | 3093delAAGA | Frameshift | Truncated protein | |
| AS3 | 16 | 3093delAAGA | Frameshift | Truncated protein | |
| AS4 | 9 | 902A → C | Missense | T106P | Mother mosaic |
| AS5 | 9 | 975T → C | Missense | I130T | |
| AS6 | 9 | 1118G → A | Missense | A178T | Polymorphism, present in father |
- * Nucleotide and amino acid numbering according to Kishino et al. [1997] (GenBank accession no. U84404).
Two disease-causing point mutations were detected, both within the first 344 bases of exon 9. In patient AS4, an A to C transversion (902A → C, Fig. 2) causing threonine to proline change at position 106 was found. The mother showed a similar but fainter abnormal CSGE band in three independent CSGE analyses, and by sequencing she was shown to be mosaic for the same T106P missense mutation (Fig. 2). The patient AS5 had a T to C transition (975T → C), which causes an isoleucine to threonine change at position 130. Neither of the parents showed the mutation. Neither of these mutations were found in the Northern Finnish control population (200 alleles studied).
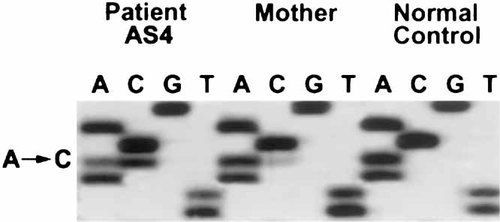
The 902A → C missense mutation identified by sequencing from patient AS4 and her mother. The fainter mutant allele in the mother's sample is indicative of mosaicism.
In patient AS6, a G to A transition (1118G → A), which causes an alanine to threonine change at position 178, was found. The same change was detected in the father and also in 1 of 100 control individuals, suggesting that the change is rather a polymorphism than causative mutation. This polymorphism has also recently been described by Matsuura et al. [1997], Malzac et al. [1997], Fang et al. [1999], and Baumer et al. [1999].
DISCUSSION
Five of the nine Angelman patients in whom deletion, UPD, and imprinting mutation had been excluded had an UBE3A gene mutation as the cause for AS. This represents 10.6% of the patients in the Finnish AS series (n = 47). This relatively high proportion correlates well with the series of Lossie et al. [2001] (10.6%), but may also be due to an increasing proportion of more complicated diagnostic cases in our laboratory.
The truncating mutations (1930delAG, 3093delAAGA) detected here were considered to be causative of AS because they lead to catalytically inactive translation products. Both the 1930delAG and the 3093delAAGA mutations have now been described in four patients [Matsuura et al., 1997; Fung et al., 1998; Fang et al., 1999; Lossie et al., 2001, this article], suggesting that these sites may be prone to deletions in the UBE3A gene. Further recurrent mutations of the UBE3A gene reported so far are a basepair insertion in exon 9 (897insA) [Russo et al., 2000], a 5 bp insertion in exon 16 (3068ins5) [Kishino et al., 1997; Malzac et al., 1997], and a C → T transition in exon 9 (2030C → T) [Malzac et al., 1997; Russo et al., 2000], which has been reported in two patients.
The two point mutations (902A → C, 975T → C) identified here have not been described earlier. Both are located in exon 9 at the p53-binding domain [Huibregtse et al., 1993b]. Although their exact effect on protein structure or function is not known, they could lead to a deficiency in the binding of substrates to be ubiquitinated and degraded. Confirmation of the mutation status of these DNA variants may need further observations of the new patients affected with AS.
The cause of AS in the remaining four patients remained unknown. Whether they represent other mutations related to the UBE3A gene or its regulation, or are caused by other possible genes, especially genes involved in the ubiquitin pathway, or whether they represent phenocopies of AS, remains to be resolved.
All the patients studied here were sporadic, although one of the mothers was mosaic for the UBE3A mutation (902A → C). So far, mosaicism for UBE3A mutations has been detected in four healthy individuals: a maternal grandfather and three carrier mothers [Malzac et al., 1997, this article]. Transmission of the mutation to the offspring indicates that it must have occurred very early in the embryo, before the differentiation of the germinal cells. It is also plausible that the mutation has occurred in the inactive paternal UBE3A allele, as a mutation in the maternal allele could be assumed to lead to phenotypic changes. So far, UBE3A mutation mosaicism concerning the maternal allele has not been described in Angelman patients, in whom it could possibly cause a clinically milder form of the syndrome. Patients who are mosaic for deletion of 15q11-q13 [Tekin et al., 2000] or non-IC-deletion imprinting defects [Buiting et al., 2003] have already been described.
To identify UBE3A mutations, sequencing of the abnormal products detected by SSCP [Kishino et al., 1997; Malzac et al., 1997; Baumer et al., 1999] or direct sequencing of amplified genomic DNA [Matsuura et al., 1997; Fung et al., 1998; Tsai et al., 1998; Fang et al., 1999; Russo et al., 2000; Lossie et al., 2001] have been used previously. Sequencing of the whole gene is the most sensitive, but a relatively expensive and laborious method, while more cost-effective screening methods have the risk of missing some mutations. SSCP is a commonly used mutation scanning technique, but its sensitivity is best with fragments of less than 200 bp, being still only 60–95% [see Eng and Vijg, 1997]. A highly sensitive and fast screening technique is denaturing high-performance liquid chromatography (DHPLC) [Xiao and Oefner, 2001], but it requires specific equipment. In this study, conformation sensitive gel electrophoresis was used to screen for mutations. CSGE is a powerful, cost-effective, and fast method with high sensitivity and specificity, reported to be close to 100% in fragments of 200–500 bp [Körkkö et al., 1998; Ganguly, 2002], and over 95% in fragments up to 800 bp [Ganguly and Prockop, 1995; Ganguly and Williams, 1997]. CSGE allowed also a more frequent use of intron primers than SSCP, which made it easier to avoid processing the UBE3AP2 pseudogene, which has high sequence homology with UBE3A [Kishino and Wagstaff, 1998].
Acknowledgements
We acknowledge the AS families for their contributions to this study, and all the physicians, who referred patients to us.




