Conjoined twins: Morphogenesis of the heart and a review
Abstract
Five cases of conjoined twins have been studied. These included three thoracopagus twins, one monocephalus diprosopus (prosop = face), and one dicephalus dipus dibrachus. The thoracopagus twins were conjoined only from the upper thorax to the umbilicus with a normal foregut. These three cases shared a single complex multiventricular heart, one with a four chambered heart with one atrium and one ventricle belonging to each twin with complex venous and arterial connection; two had a seven chambered heart with four atria and three ventricles. The mono-cephalus diprosopus twins had a single heart with tetralogy of Fallot. The dicephalus twins had two separate axial skeletons to the sacrum, two separate hearts were connected between the right atria with a shared inferior vena cava. Thoracopagus twinning is associated with complex cardiac malformations. The cardiac anlagen in cephalopagus or diprosopus are diverted and divided along with the entire rostral end of the embryonic disc and result in two relatively normal shared hearts. However, in thoracopagus twins the single heart is multiventricular and suggests very early union with fusion of the cardiac anlagen before significant differentiation. Cardiac morphogenesis in conjoined twins therefore appears to depend on the site of the conjoined fusion and the temporal and spatial influence that determines morphogenesis as well as abnormally oriented embryonic axes. © 2003 Wiley-Liss, Inc.
INTRODUCTION
Conjoined twins of approximately equal size are incompletely separated twins but retain a degree of overall symmetry no matter what the pattern of “fusion.” Cardiac morphogenesis in conjoined twins appears to depend on the site of fusion and the orientation of the embryonic axes.
We have examined five cases of conjoined twins with particular reference to the development of the cardiovascular system. We will report these cases, briefly review the classification of conjoined twins and discuss cardiac morphogenesis in this form of twinning. Conjoined twinning is a defect of blastogenesis and has attracted exceptional attention by professionals and lay persons since antiquity. Albrecht Dürer, the greatest artist of the German late medieval-early Renaissance period, portrayed with exceptional clarity the “Siamese twins of Erhlingen” in 1512. Clearly female and separate to the upper pectoral girdle, they had a single umbilical cord, an apparently normal single lower body, a normal right and left upper limb, and posteriorly an upper limb “fused” to the elbow, with distal branching of separate forearms and hands. A few other celebrated conjoined twins will be cited to illustrate the extraordinary complexity of these gemellus defects of blastogenesis.
CLINICAL REPORTS
CASE 1
The twins, females, were born to a G2P1, 16-year-old African American woman. The pregnancy was uncomplicated. There was no history of incest or of viral infection, ingestion of drugs, or exposure to radiation during pregnancy. The maternal grandfather was a twin and a maternal aunt had given birth to normal twins.
Birth occurred after 38 weeks of gestation. The infants were of approximately equal size and their combined weight was 3.4 kg.
The heads were hyperextended and both twins had severe lordosis. They were conjoined along the midline of the thorax to the umbilicus, which was single. All reflexes were present and each voided and passed meconium spontaneously. The heart sounds were diffuse with a rate of 124 per min. No heart murmurs were detected. Although femoral pulses were palpable, those of twin B were weak. A midline liver could be palpated 1 cm below the costal margin. The genitalia were normal. During the first 24 hr of life mild respiratory distress was noted with respiratory rates of 50 per min.
Upper gastrointestinal radiographs demonstrated two separate stomachs and a common proximal small bowel. Electrocardiograms demonstrated identical heart rates, but the complexes were different. Two weeks after admission signs of pneumonia developed. The infants did not respond to antibiotic therapy and died at age 19 days.
Pathological Description
The twins were fused from the level of the manubrium sterni to just below the umbilicus (Fig. 1). The vertebral columns were normal except for prominent thoracolumbar lordosis, more prominent in twin A.
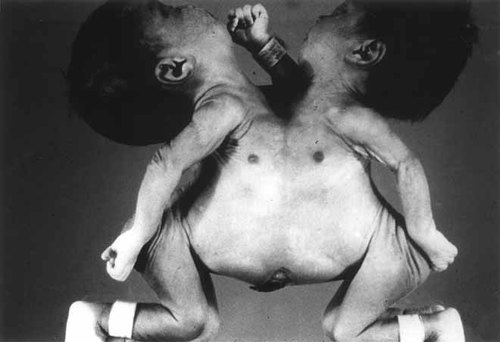
Case 1: Thoracopagus conjoined twins at 38 weeks of gestation.
The rib cages were conjoined in the frontal plane. The manubria were fused, forming an arch, while the sternal body and xiphoid were bifid in each twin. The right sternal segment of one twin was fused to the left sternal segment of the other and vice versa. This sagittal split in the otherwise conjoined sterna allowed the common pericardial sac and heart to develop.
The superior portion of the mediastinum was separate and normal, but the lower part was conjoined at the common pericardial sac, which was closely adherent to both common sterna in its entire length and separated the common thorax into two parts, each belonging to one of the twins.
Pathological Diagnosis
- I
Thoracopagus conjoined twins
- A
Fused manubrium sterni
- 1
Bifid sternal bodies
- 2
Bifid xiphoid processes in each twin
- 1
- B
Single shared thoracic cavity with common lower mediastinum.
- C
Single pericardial sac containing four-chambered fused heart with one atrium and one ventricle belonging to each twin (Fig. 2).
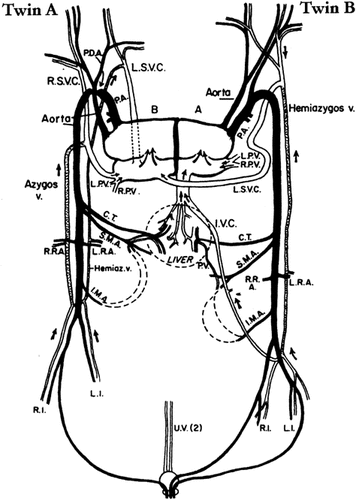
Case 1: Diagram of cardiovascular system of conjoined twins; black solid lines, arterial system; dotted lines, azygos system. RSVC, right superior vena cava; LSVC, left superior vena cava; IVC, inferior vena cava; PA, pulmonary artery; RPV, right pulmonary vein; LPV, left pulmonary vein; CT, celiac trunk; SMA, superior mesenteric artery; IMA, inferior mesenteric artery; RRA, right renal artery; LRA, left renal artery; PV, portal vein; RI, right iliac vessels; LI, left iliac vessels; UV, umbilical veins; PDA, patent ductus arteriosus.
- i
Twin A
- 1
Both great arteries originate from a single ventricle (aorta lateral and slightly anterior to pulmonary artery).
- 2
Single atrioventricular valve with three leaflets.
- 3
Coarctation of aorta, mild.
- 4
Right brachiocephalic, left common carotid, and left subclavian arteries originate from arch of aorta: separate origin of left common carotid and left subclavian arteries: right brachiocephalic artery gives origin to right subclavian, right internal carotid, and right external carotid arteries.
- 5
Absent left umbilical artery.
- 6
Patent ductus arteriosus.
- 7
Anomalous systemic venous return: absent inferior vena cava; right superior vena cava receives innominate, azygous and jugular veins; persistent left superior vena cava receives hemiazygous vein and enters atrium at site of large dilated coronary sinus; four major hepatic veins from common liver plus inferior vena cava of twin B and from large vein entering atrium of twin B.
- 1
- ii
Twin B
- 1
Origin of aorta and pulmonary artery from single ventricle (aorta medial and slightly anterior to pulmonary artery).
- 2
Single atrioventricular valve with three leaflets.
- 3
Complete interruption of aortic arch with brachiocephalic and left common carotid arteries originating from ascending aorta.
- 4
Widely patent ductus arteriosus.
- 5
Right subclavian artery originates from junction of ductus arteriosus and descending aorta.
- 6
Anomalous systemic venous return: absent right superior vena cava; persistent left superior vena cava (receiving hemiazygous, innominate, and jugular veins) with drainage into atrium of twin A: absent azygous vein.
- 7
Inferior vena cava receives four hepatic veins and empties into atrium of twin B.
- D
Single shared abdominal cavity with common diaphragm.
- E
Gastrointestinal malformations.
- 1
Union of duodenums at second portions, forming a common duodenum with single ampulla of Vater receiving single common bile duct.
- 2
Common jejunum.
- 3
Common proximal ileum with subsequent division into two terminal ilea at level of remnant of vitello-intestinal duct: bowel of twin A dilated, of twin B extremely small.
- 4
Two separate mesenteries for common portion of small bowel with separate arterial and venous blood supply.
- F
Semiannular pancreas for each twin with head of each pancreas overlying anterior non-fused portion of each duodenum.
- G
Single common liver with fusion of anterior and posterior portions at porta hepatis.
- H
Two separate gallbladders and cystic ducts, one each to anterior and posterior portions of liver. Union of one hepatic duct from anterior portion of liver and one hepatic duct from posterior portion forming single common bile duct draining into ampulla of Vater, in second portion of common duodenum.
- I
Lordosis at thoracolumbar level—severe in one twin, moderately severe in other.
CASE 2
The delivery of these stillborn Caucasian, female twins was induced at 21 weeks of gestation. This was the mother's first pregnancy. An initial ultrasound study showed twins joined from the thoracic to the umbilical region.
A repeat ultrasound study confirmed that the conjoined twins shared a common heart and a common liver. There were also bilateral cystic hygromas.
Pathological Description
These twins weighed 1,020 g and were joined from the thorax to the umbilicus. Twin B had a cleft lip but an intact palate (Fig. 3). The genitalia were female. All upper and lower limbs were normally developed. A 3-cm defect was present in the abdominal wall, immediately beneath the insertion of the umbilical cord, with herniation of the large intestines through this defect representing an apparent gastroschisis. There was a fused rib cage with single sternum.

Thoracopagus conjoined twins at 21 weeks gestatin (Case 2).
Heart
The single heart (Fig. 4A,B) consisted of seven chambers. The systemic venous drainage of twin A was normal with a right superior vena cava and left innominate vein. The systemic venous drainage of twin B was via a persistent left superior vena cava returning to the coronary sinus which entered a left-sided morphologically right atrium. There were two morphologically right atria on the anterior aspect of the heart and two morphologically left atria on the posterior aspect. Twin A had normal atrial situs and twin B atrial situs inversus. The left-sided morphologic right atrial appendage of twin B was hypoplastic. The inferior vena cava left the right atria as a single vessel and subsequently divided as it entered the shared liver. Twin A had a hypoplastic but patent foramen ovale. Twin B had a very large foramen ovale that encompassed approximately half of the total atrial septum. The flap valve was somewhat redundant and fenestrated. Adjacent to it was a markedly dilated coronary sinus where the persistent left superior vena cava (twin B) returned. A coronary sinus was not identified in twin A. There was an atrial septal defect of the primum type just beneath the large foramen ovale and large coronary sinus. Both right atria entered through a single inlet into a shared atrioventricular valve and morphologic right ventricle. The atrioventricular valve was consistent with a tricuspid valve, having both papillary muscle and septal attachments. Each twin had its own outlet chamber that communicated with the central, shared ventricle via separate muscular ventricular septal defects. The aorta arose immediately anterior to the pulmonary artery on twin A. The pulmonary artery was stenotic and the ductus arteriosus was very thin and tenuous. The great vessels branched normally from the arch. The aorta of twin B lay side by side and to the right of the pulmonary artery. There was absence of the ductus arteriosus in twin B and there was distal origin of the right subclavian artery. The carotid arteries branched separately from the aortic arch. The pulmonary venous return of twin A was anomalous, the veins from each lung joining to form a single vessel that extended below the diaphragm, draining into the portal vein. Twin B had normal pulmonary venous return. The twins shared a single liver that appeared to represent the fusion of two morphologically normal structures. Two gallbladders were present in the right lobe of each portion of the liver. The gastrointestinal tract was separate from the esophagus to the distal duodenum where the two segments joined as a single jejunum. At the terminal ileum the bowel separated, each twin with a separate cecum, appendix, and large bowel. Each twin had a structurally normal uterus with adnexae. The urinary tracts were separate and morphologically normal.
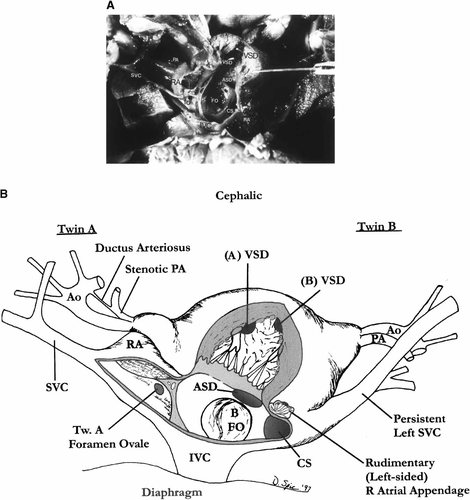
Case 2. A: Gross appearance of the opened common heart. B: Diagram of the heart. There is a common ventricle with a rudimentary outlet chamber for each twin. ASD, arterial septal defect (primum type); VSD, ventricular septal defect; SVC, superior vena cava; RA, right atrium; IVC, inferior vena cava; FO, foramen ovale; CS, coronary sinus; PA, pulmonary artery; Ao, aorta.
The placenta was monoamniotic, monochorionic and weighed 300 g. The umbilical cord extended from the abdominal region of the twins and inserted centrally on the fetal surface. Four umbilical vessels were identified, two umbilical veins and two umbilical arteries.
Pathological Diagnosis
- I
Thoracoomphalopagus female twin fetuses at 21 5/7 weeks of gestation.
- A
Single heart
- 1
Seven chambers
- 2
Normal atrial situs-Twin A
- 3
Hypoplastic foramen ovale-Twin A Large foramen ovale with fenestrated flap valve-Twin B
- 4
Atrial septal defect, primum type
- 5
Persistent left superior vena cava draining to a large coronary sinus-Twin B
- 6
Common morphologic right ventricle with two muscular ventricular septal defects, one communicating with the outflow chamber of each twin.
- 7
Pulmonary stenosis-Twin A
- 8
Stenotic ductus arteriosus-Twin A Absent ductus arteriosus-Twin B
- 9
Distal origin of the right subclavian artery-Twin B
- 10
Total anomalous pulmonary venous return-Twin A
- 1
- B
Single shared liver
- C
Shared jejunal segment of the small bowel
- A
- II
Cleft lip-Twin B
- III
Second trimester monoamniotic monochorionic placenta with four vessel umbilical cord, lacking the left/right umbilical artery in twin A/B.
CASE 3
The mother was a 30-year-old woman who was diagnosed by ultrasound to have conjoined twins. She underwent an elective dilatation and evacuation (D&E). Records indicate that the pregnancy was at about 12 weeks of gestation. Ultrasonography had indentified a single heart and umbilical cord and a shared liver.
Pathological Description
There were multiple small fragments of fetal tissue and organs and no intact fetuses; the feet were 10 mm long. A fused malformed rib cage and two separate spinal columns were identified. The orientation of the twins appeared to be face to face and joined from the upper sternum down. A single malformed heart and a single umbilical cord with three vessels were identified. The sex of these fetuses could not be determined.
Heart
Attached to the heart (Fig. 5) were irregular somewhat shaggy tan-white tissues that appeared to represent pericardium and lung tissue. Apparent right and left superior venae cavae were identified and appeared to enter the right atrium to each twin. Two other venous structures were identified near the superior vena cava on each side and appeared to enter the left atrium of each twin. These appeared to represent pulmonary veins. On the anterior aspect there were two morphologically right atria with two morphologically left atria on the posterior aspect. Twin A had normal atrial situs and twin B had atrial situs inversus. Each twin had a recognizable aortic and pulmonary trunk exiting the ventricular mass. A ductus arteriosus was not identified on twin B and on twin A a very thin and tenuous ductus arteriosus was present. There was a communication between the morphologic right atrium of each twin, giving the appearance of a common chamber. There was a patent foramen ovale in both twins. Twin B had a single ventricle that had a single inlet and a double outlet. Its morphology was that of a right ventricle. Twin A had a large ventricular chamber that was a morphologic right ventricle and a smaller rudimentary outflow chamber that appeared to be of left ventricular morphology. There was an atrioventricular septal defect between the large morphologic right ventricle and the rudimentary outflow chamber. Twin A had a double-outlet right ventricle. Between the morphologic right ventricles of twin A and twin B, there was a muscular ventricular septal defect. The pulmonary venous drainage could not be assessed. An illustration of the heart is shown in Figure 6.
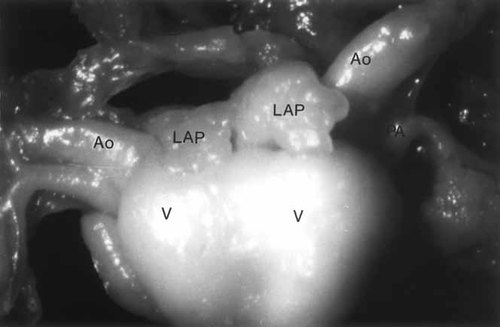
Case 3: Thoracopagus conjoined twin heart from dilatation and evacuation specimen at 12-weeks gestation taken under dissecting microscope. Ao, aorta; PA, pulmonary artery; LAP, left atrial appendage; V, ventricle (three separate ventricles were identified).
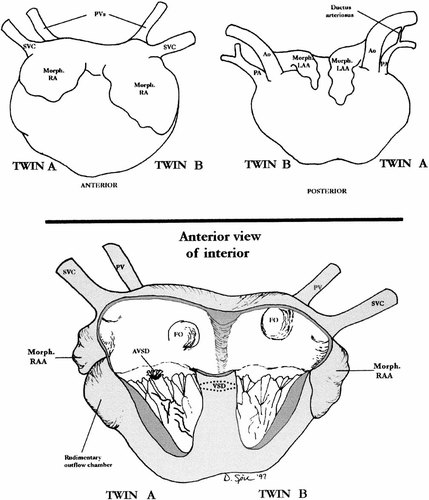
Case 3: Illustration of common heart. SVC. superior vena cava; PV, pulmonary vein; FO, foramen ovale; RAA, right atrial appendage; AVSD, atrioventricular septal defect; VSD, ventricular septal defect.
Pathological Diagnosis
- I
Thoracopagus conjoined twins at ∼12 weeks of gestation.
- A
Single shared heart
- 1
Seven chambers
- 2
Normal atrial situs-Twin A Atrial situs inversus-Twin B
- 3
Atrioventricular septal defect-Twin A
- 4
Double outlet right ventricle-Twin A and Twin B
- 5
Muscular ventricular septal defect between the two large ventricular chambers of Twin A and B
- 6
Stenotic ductus arteriosus-Twin A
- 7
Absent ductus arteriosus-Twin B
- 8
Single ventricular chamber-Twin B
- 1
- B
Only intact organ received was the heart.
CASE 4
The mother was a 23-year-old G2P1 woman who presented at 16 weeks with fetal death in utero. The fetus was delivered spontaneously. The fetus was attached to the umbilical cord and placenta and weighed 50 g; the placenta weighed 90 g. The umbilical cord measured approximately 6 cm between the fetal umbilicus and insertion onto the placental disc. Foot length was 1.8 cm, crown rump length 7.0 cm, and crown heel length 10.0 cm, corresponding to an estimated gestational age of 15–16 weeks.
Pathologic Description
This was a janiceps anencephalic male fetus with complete craniorachischisis extending to ∼1.5 cm above the anus (Fig. 7A). The heads were fused at the midline and each contained two eyes, one nose, one ear, and one mouth. The two ears were low set (Fig. 7B). The eyes were protuberant. The heads were flexed posteriorly and laterally. The calvarial bones were absent. The brain was replaced by a mass of vascularized neuroglial tissue. Two separate spinal cords were identified. The upper limbs were unremarkable. The chest appeared unremarkable. The neck was flexed posteriorly. The abdomen was distorted by a large omphalocele containing the entire liver, a portion of large and small bowel including the appendix, stomach, and spleens. These were covered by a thin membrane, and a trivascular umbilical cord arising from the dome of the omphalocele. The external genitalia were male. The lower limbs appeared unremarkable.
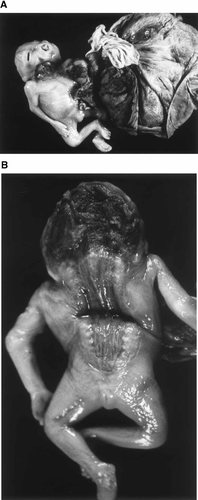
Case 4: Monocephalus diprosopus twins with attached placenta. A: Anterior view with short cord and omphalocele. B: posterior view showing anencephaly with craniosacral rachischisis.
A single midline thymus was identified. The thorax contained bilobed right and left lungs with hyparterial bronchi. On the right there was a pulmonary sequestration immediately beneath the lower lobe. The sequestration had a separate arterial supply that extended from the posterior aspect of the aortic arch along the spine to this extra lobe. The pulmonary venous return extended below the diaphragm and drained into a gastric vein. A single heart was in the midline and there was tetralogy of Fallot. The aorta arched to the left and descended along the midline into the abdominal cavity. The vessels branching from the arch were as follows: right innominate artery branching to a right subclavian and right carotid artery and a left innominate artery that branches to a left subclavian and left carotid artery. A proximal trachea extended from each pharynx. An esophagus extended from the palate of each twin and formed a fused Y-shaped hollow viscus extending into a single dilated midline stomach. The diaphragm was represented by a thin transparent membrane. The liver was entirely contained in the omphalocele sac and was misshapen; the gallbladder was absent. There was bilateral polysplenia with multiple splenic lobules on both the right and left aspects of the midline stomach (Fig. 8). There was a single very short small intestine. The appendix was identified and there was a single large bowel. A small nodule of firm yellow-white pancreatic tissue was identified posterior to the stomach. The right kidney and adrenal were in their normal position. The left kidney lay in the midline and the left adrenal was anterior to it and stretched into the omphalocele sac. The ureters extended into the bladder and testes were identified in the pelvis.
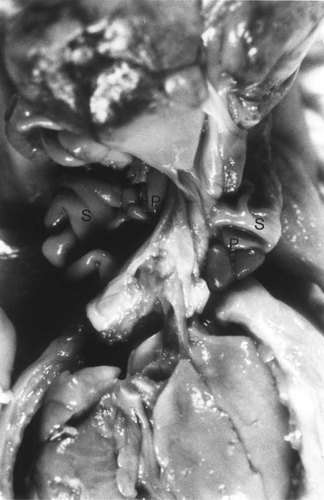
Case 4: Abdominal cavity opened showing bilateral polysplenia adjacent to a large shared midline stomach. P, polysplenia; S, stomach.
The placenta measured 10 × 9 × 1 cm and showed no abnormalities. The umbilical cord was eccentrically located with four vessels—two umbilical veins and two umbilical arteries. The membranes were translucent and smooth.
Pathological Diagnosis
- I
Fetus:
- A
Monocephalous diprosopus, (janiceps) dibrachus, dipus conjoined twins with anencephaly and complete craniorachischisis.
- B
Notochord axes side by side with two axial skeletons.
- C
Congenital heart disease
- 1
Tetralogy of Fallot
- 2
Bilateral innominate arteries.
- D
Polysplenia, bilateral
- E
Bilateral bilobed lungs with hyparterial bronchi
- F
Pulmonary sequestration-right
- G
Omphalocele with liver, large bowel, small bowel, including appendix, stomach, and spleen contained within omphalocele sac.
- H
Duplication of proximal trachea, extending into hypopharynx.
- I
Duplication of proximal esophagus, extending into hypopharynx.
- J
Hypoplastic diaphragm, represented by thin translucent membrane.
- A
- II
Monoamnionic monochorionic placenta with trivascular umbilical cord.
- A
Short umbilical cord, 6 cm in length.
CASE 5
The fetus was the product of a spontaneous abortion of a G1, 25-year-old white mother. The fetus was a dicephalus, dibrachus, dipus conjoined twin.
Pathological Description
The two heads appeared grossly unremarkable with normal appearing ears, eyes, nose, and mouth (Fig. 9). The lip and palate of each twin were intact. The necks appear normal and are angled slightly from the joined shoulder, thorax and abdomen. The thoracic and abdominal cavities were shared. Twin A had a normal appearing upper and lower right limb each with five digits. Twin B had a normal appearing left upper and lower limb with five digits on each. The twins weighed 52 g. The crown rump length was 9.4 cm and the foot length was 1.4 cm, these measurements were consistent with an estimated gestational age of 14–15 weeks. Twin A had a left clavicle and twin B a right clavicle; these were deviated cephalad and formed a triangular projection between the two heads. The genitalia were male and the anus was patent. There was complete separation of the vertebral columns (Fig. 9A,B) extending to a single pelvis. The ribs were normal along the lateral aspect of the thoracic cavity, there were two sternums and multiple shared ribs in the midline.
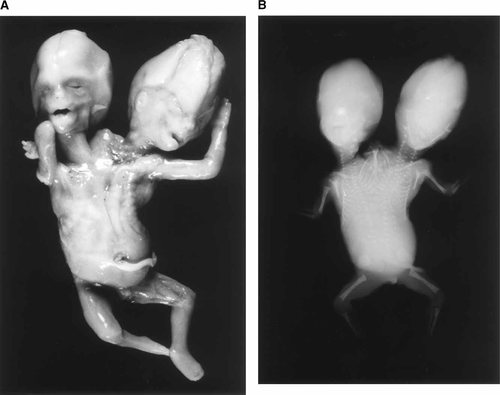
Case 5: Dicephalus dibrachus dipus twins. A: anterior view. B: X-ray showing two axial skeletons.
There was a single, three-vessel umbilical cord with symmetric single midline liver and absent gallbladder. Each twin had its own esophagus, stomach, duodenum, and proximal jejunum. The duodenum and proximal jejunum each had their own mesentery and the distal jejunum and ileum joined to give the appearance of an intestinal duplication. This extended to the distal ileum. At the distal ileum the bowel became a single lumen, with a single cecum, appendix, and large bowel. Twin B had a normal spleen and twin A had an absent spleen. The stomach of twin A was on the right. There was a midline shared pancreatic head with a pancreatic tail extending to the right of twin A and to the left of twin B. The pancreas was annular in twin A with duodenal stenosis. Two kidneys and two adrenals were present, both of which appeared normal. Two testes were identified in the abdominal cavity. The diaphragm was intact (Fig. 10).
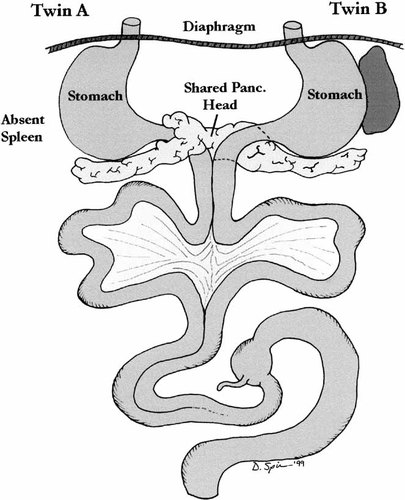
Case 5: Illustration of abdominal contents.
Twin A had bilaterally trilobed lungs with bilateral eparterial bronchi. The lungs of twin B had a normal situs. There was a shared midline pleural cavity containing the left lung of twin A and the right lung of twin B. The heart was shared, but only one right atrium (Fig. 11). This common right atrium was in the midline and gave rise to a left superior vena cava in twin A and a normal right superior vena cava in twin B. The inferior venae cavae drained separately into the liver, on the left in twin A and on the right in twin B. Twin A had a persistent left superior vena cava draining to the coronary sinus and there was total anomalous pulmonary venous return to the right atrium. The apex of the heart pointed to the right and there was atrial situs inversus. The foramen ovale was patent and there was an atrioventricular septal defect. There was transposition of the great arteries; the large anterior aorta left the morphologically right ventricle. The aorta arched to the right and there was mirror-image branching of the arteries as they left the aorta. There was a right ductus arteriosus. The cardiac and abdominal findings in twin A were consistent with asplenia.
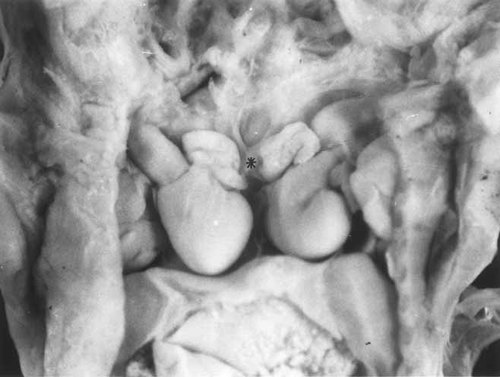
Case 5: Opened thoracic cavity with two separate hearts joined by a common right atrium (*).
An illustration of the heart is shown in Figure 12. The Apex of the heart in twin B pointed to the left and there was a normal atrial situs. The foramen ovale was patent and the atrioventricular and ventriculoarterial connections were normal.
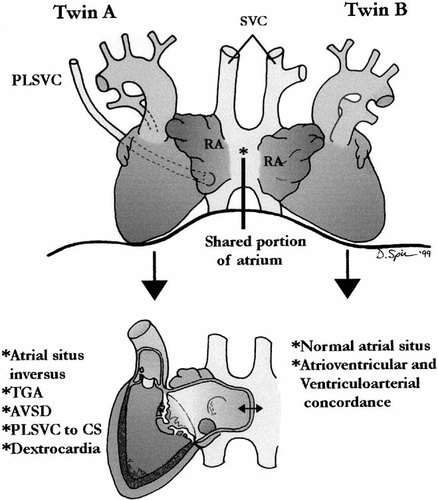
Case 5: Illustration of heart showing normal heart (Twin B) and complex congenital anomalies n Twin A with asplenia SVC, superior vena cava; RA, right atrium (* junction connecting the atria).
Pathological Diagnosis
- I
Fetus:
- A
Dicephalus, dibrachus, dipus conjoined twins.
- B
Shared heart—one common right atrium.
- 1
Twin A—complex congenital heart disease and findings of asplenia.
- (a)
Transposition of the great vessels with pulmonary stenosis
- (b)
Atrioventricular septal defect
- (c)
Atrial situs inversus
- (d)
Left-sided superior and inferior vena cava
- (e)
Absent left innominate vein
- (f)
Persistent left superior vena cava draining to the coronary sinus
- (g)
Total anomalous pulmonary venous return to the right atrium
- (h)
Right aortic arch with mirror-image branching
- (i)
Right-sided ductus arteriosus
- (j)
Dextrocardia
- (k)
Bilateral trilobed lungs with eparterial bronchi
- (a)
- 2
Absent spleen
- 3
Right-sided stomach
- 4
Annular pancreas around the duodenum with a pancreatic tail extending to the right.
- C
Twin B normal cardiac anatomy
- D
Multiple shared ribs
- E
Shared central pleural cavity
- F
Central pancreatic head with a tail extending to each twin
- G
Single, symmetric liver
- H
Absent gallbladder
DISCUSSION
Perhaps the first recorded conjoined twins in Western history were the Biddenden maids in England who were probably pyopagus twins. “Cakes” (flour and water) were imprinted with their image and were passed out to the poor once a year, using funds from their bequest of land to the local church when they died in 1134. Their shoulders are always shown close together, but that probably simply represents the fact that they walked with their arms around each other. (Fig. 13)

Biddenden twins circa 1100 AD. This reproduction is from an imprinted cake from Ballantyne's book on Antenatal Pathology 1904 (Ballantyne JW: Manual of Antenatal Pathology and Hygiene, Edinburgh, William Green, 1904).
The original “Siamese twins” of the 19th century were Chang and Eng who were brought to the USA by a sea captain after observing them living on a houseboat in Bangkok when they were 18 years old. (Fig. 14) They joined the Barnum & Bailey Circus in Baraboo, Wisconsin, and later married twin girls and lived in Virginia where they fathered 22 children. Other historical and remarkable cases of conjoined twins have been described including the Blazek twins (Josepha and Rosalie) from Bohemia (Fig. 15) who gave birth to an infant through a single vagina, but gestation had occurred in the uterus of one of the twins. More recently the Hensel twins are the only presently living examples of conjoined twins.
Change and Eng Bunker from an early description by J.C. Warren in 1829 (Warren JC: An account of the Siamese twin brothers united together from their birth. Am J Med Sci 52:255, 1829).

Blazek twins, illustrations taken from textbook of Embryology by Broman 1911 (Broman I: Normale and Abnorme Entwickland des Menschen Wiesbaden, Bergmann, 1911).
Conjoined twins are the result of incomplete separation of a single ovum, hence, monozygotic twins. The reported incidence of conjoined twins ranges from 1:192,000 births in southern Africa, through 1:33,000 to 1:165,000 in North America, [Rudolph et al., 1967; Benirschke and Kim, 1973; Myrianthopoulos, 1975; Edmunds and Layde, 1982], 1:100,000 in Japan, [Imaizumi, 1988] 1:75,000 in Sweden [Källén and Rybo, 1978], 1:68,000 in Hungary [Métneki and Czeizel, 1989]. Nine cases of conjoined twins as part of a triplet set have been described [Tan et al., 1971]. These variations reflect the relative proportion of monozygotic twins in these populations.
There is a striking female preponderance of up to 90–95% of the reported cases [Milham, 1966; Potter and Craig, 1975]. This excess of females may be related to the pathogenesis of the anomaly related to the mechanisms of monozygotic twinning and its relation to a delay of fertilization in female zygotes.
The actual pathogenetic mechanisms have yet to be defined. Events that occur earlier in embryogenesis, either incomplete fission of the developing embryo or the development of codominant axes with apposition of part of the axes that leads to conjunction, have been suggested [Levin et al., 1996].
The conjunction of the heart of conjoined twins is usually the limiting factor to survival and the consideration of surgical separation. We have studied five cases of conjoined twins with particular emphasis on the cardiovascular system and the association with laterality defects.
| The classification of conjoined twins includes: | |
| Katadidyma | Single in the lower body and double above |
| Diprosopus | 2 faces side by side with 1 head and 1 body |
| Dicephalus | 2 heads and necks side by side with 1 body |
| Ischiopagus | Joined by the inferior margins of the coccyx and sacrum; 2 completely separate spinal columns |
| Pyopagus | Joined by the lateral and posterior surfaces of the coccyx and sacrum; these twins are back to back |
| Anadidyma | Single in the upper portion of the body and double below |
| Dipygus | 1 head, thorax, and abdomen with 2 pelves with or without 2 sets of external genitalia and up to 4 legs |
| Syncephalus | Joined by the face; the faces are turned laterally |
| Craniopagus | Joined at the cranial vault |
| Anakatadidyma | Joined by the midportion of the body and separate and double above and below the region possessed in common |
| Thoracopagus | Part of the thoracic wall and contained viscera is common to both twins, face to face |
| Omphalopagus | Joined from the umbilicus to the xiphoid, face to face |
| Rachipagus | United at the vertebral column at any point above the sacrum, back to back |
Conjoined twins have a high mortality rate. Nearly 40% are stillborn and a further one third die within 24 hr of birth [Baldwin, 1994]. The causes of death are usually related to the severely abnormal conjoined heart or to the pulmonary hypoplasia that accompanies the distortion of fused thoracic cages.
It has been suggested that conjoined twins arise from ectopic primitive streaks. It has been found that parallel primitive streaks allow activin cross-signaling to the right and show normal sonic hedgehog (Shh) expression in the left embryo and lack of expression in the right embryo. When hybridized to a nodal probe, there is normal nodal expression in the left embryo and lack of expression in the right embryo that may result in inverted heart situs [Levin et al., 1996].
Oblique twin streaks show normal nodal expression in the right embryo and double nodal expression in the left embryo [Levin et al., 1996].
In craniopagi and ischiopagi, the primitive streaks are end-to-end and signaling molecules do not affect each other. However, in dicephali and thoracopagi, the primitive streaks are adjacent to each other and allow cross-signaling with consequent laterality defects.
THORACOPAGUS TWINS
The most common pattern of conjoined twins is face to face fusion of the thorax and variable portions of the abdomen-thoracopagus [Filler, 1986].
One of the most critical factors to survival or separation of thoracopagus twins is the degree of cardiac fusion. Although cardiac fusion is present in 75% of cases [Marin-Padilla et al., 1981], the degree of fusion cannot be predicted by the degree of thoracic fusion [Singer and Rosenberg, 1967]. A common pericardium is observed in 90% of cases and atrial fusion is almost always present if any cardiac fusion exists at all.
Many of the anomalies in these twins seem to be manifestations of alterations in body symmetry [Singer and Rosenberg, 1967]. The normally asymmetrical structures (heart and great vessels, lungs, liver, spleen, and gastrointestinal tract) are rendered more symmetrical. The normally symmetrical structures (brain, upper respiratory tract, urogenital system, and skeleton) are less severely affected or not at all. The degree of fusion of other viscera varies. The liver is shared in all cases but the intestinal tract is shared in only about half of the cases. There may be an omphalocele at the site of the conjoined umbilicus [Milham, 1966].
Thoracopagus twins are thought to result from union of the cardiac analagen and the septum transversum out beyond the rostral edge of the very early embryonic disc. Subsequent development of the headfolds permits normal evolution of the entire head and neck, including the brain and the face. Only the chest wall is involved in division and diversion, resulting in one large thorax with shared “anterior” and “posterior” sterna, each receiving ribs from each twin. Typically, there is one large complex heart with multiple ventricles and duplicate aortic arches (or trunci arteriosi). The most rostral portion of the foregut is not involved, but the duodenum is frequently conjoined. The two livers usually show minimal to moderate ventral union but in some instances are fused into one large mass [Spencer and Robichaux, 1998].
OMPHALOPAGUS TWINS
These are the most readily separable conjoined twins since their union may involve only skin and portions of liver (as in Chang and Eng Bunker), occasionally including portions of the sternum [Nichols et al., 1967].
CEPHALOTHORACOPAGUS TWINS
In cephalothoracopagus twins, the cranial fusions may be lateral with a single distorted facial zone, or the double facial zones on either side as in the janiceps type [Herring and Rowlatt, 1981]. It has been suggested that each face in the janiceps type is made up of the right side of one twin and the left side of the other [Guttmacher and Nichols, 1967]. The degree of conjunction and deformity depends on the extent of fusion of the trunks [Wedberg et al., 1979; Delprado and Baird, 1984; Merwin and Wright, 1984; Baron et al., 1990]. The thoracic and abdominal organs are often duplicated. The heart and anterior intestinal derivatives tend to be oriented anteroposteriorly and to be shared by the twins rather than belonging to the right or left individual. The posterior organs tend to maintain a right and left orientation.
OMPHALOPAGUS TWINS
Omphalopagus twins have two separate hearts, perhaps with an avascular band between the atria or ventricles. The distinction between thoracopagus and omphalopagus is the uniform fatality of the former and the frequent survival of the latter [Spencer and Robichaux, 1998].
CRANIOFACIAL DUPLICATIONS
Craniofacial duplications are unusual in two respects. First, they are frequently associated with neural tube defects [Gupta, 1966; Herring and Rowlatt, 1981]. Second, while there is a spectrum of facial duplication from isolated nasal duplication to the complete doubling of all facial structures as in diprosopus twins [Chervenak et al., 1985], it is questionable whether this type of anomaly is truly a conjoined twin or merely a regional duplication for some other reason.
The cardiac anlagen in cephalopagus are divided and diverted (along with the entire rostral end of the embryonic disc), usually resulting in two relatively normal shared hearts, each under a different shared sternum. The single heart in thoracopagus is typically multiventricular, strongly suggesting that very early union with intermixing of the cardiac anlagen occurs before any significant differentiation takes place [Spencer and Robichaux, 1998].
Our Case 5 is similar to the Hensel twins who have been reported widely in the “popular press,” but to our knowledge not in the scientific literature [q v Life, April, 1996].
PATHOGENESIS OF LATERALITY DEFECTS IN CONJOINED TWINS
Gene expression in twin chick embryos has provided insight into the pathogenesis of laterality defects in human conjoined twins [Torgensen, 1949; Aird, 1959; Curniff et al., 1988]. Levin et al. [1996] have studied 167 pairs of conjoined twins. They concluded that the propensity towards laterality defects depends on the orientation of the conjoined twins. Where the twins were joined obliquely at the chest and/or abdomen (thoracopagi) or laterally at the chest (dicephali), one of the twins had reversal in heart situs; however, none of the twins joined only at the head (craniopagi) or pelvis (ischiopagi) exhibited laterality defects. When laterality defects are present, they occur most frequently in the twin on the right side of dicephalus (86%) and thoracopagus (71%) twins.
In the mouse model activin, on the right side of the primitive streak, represses expression of the gene Sonic hedgehog (SHH) on the right. The remaining expression of Shh on the left is responsible for inducing nodal on the left, ultimately specifying heart situs. When twins arise from two parallel primitive streaks, the activin produced on the right side of the left embryo may inhibit the expression of SHH in the left side of the right embryo [Goldstein et al., 1998] (Fig. 16).
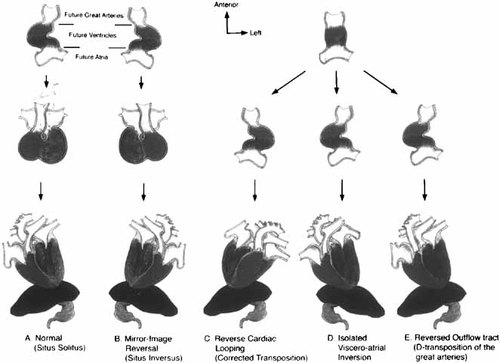
Model for how left–right reversals affecting different segments might perturb anatomy. A: Represents the normal rightward looping of the heart tube and normal cardiac position. B: Shows mirror-image reversal, with leftward looping and dextrocardia, presumably due to inversion of the left–right signals. C: There is a leftward looping of the cardiac tube; however, normal physiologic circulation results, due to L-transposition of the great vessels. D: Represents a form of heterotaxy, with isolated atrial and visceral inversion. E: Normal cardiac looping occurs, but the great vessels have been reversed, resulting in normal atrioiventricular and reversed ventriculoarterial connections. Courtesy of Goldstein et al. [1998] and Dev Genet.
If the twin primitive streaks form at an angle rather than being completely parallel, the two nodes become closer together as the streaks elongate. In some instances, the repression of Shh by activin will proceed normally because the streaks will initially be too far apart for the signals to diffuse. Double-sided nodal expression leads to randomization of its situs in obliquely oriented spontaneous twin embryos [Goldstein et al., 1998].
In craniopagi and ischiopagi twins, the primitive streaks are end-to-end, and signaling molecules to the side of one would not be expected to affect the other. However, in dicephali and thoracopagi twins, the streaks positioned adjacent to each other allow cross-signaling.
Activin, SHH and nodal normally act only on the side of the embryo where they are expressed [Levin et al., 1996].
LEFT–RIGHT AXIS ASYMMETRY
Abnormalities in left–right (LR) asymmetry is based on the prediction that these reflect disturbances in signaling to the heart during development [Goldstein et al., 1998]. The notochord and neural tube appear to be important signaling centers that contribute LR information to the developing heart. This predicts that LR cardiac malpositions might be accompanied by congenital defects of the axial skeleton and neural tube.
There are several molecules that transiently exhibit obvious LR asymmetries. Many of these, including nodal, sonic hedgehog, activin [Levin et al., 1995], lefty [Meno et al., 1996], and Vg1 [Hyatt et al., 1996] are implicated in guiding looping of the heart.
One important cardiac gene critical to the interpretation of extracardiac signals is BMP4. Symmetric when the heart-tube first forms, BMP4 rapidly becomes left predominant [Chen et al., 1997]. BMP4 may participate in interpreting the extracardiac LR patterning system to morphogenetic movements within the heart necessary for proper LR asymmetry.
Mouse models of left–right asymmetry offer a powerful opportunity for dissecting the genetic and embryological basic of laterality.
A mouse mutant in the activin receptor type IIB (ActRIIB) gene shows randomization of the positioning and apical orientation of the heart, in addition to bilateral right-sidedness of the pulmonary and atrial anatomy [Oh and Li, 1997], findings resembling the asplenia syndrome in humans. ActRIIB−/− mice also manifest abnormalities of the axial skeleton with additional thoracic vertebrae, anterior transformation of multiple vertebrae, and altered expression of HOX genes along the AP axis. This is compatible with a role for the midline in directing LR asymmetry. Several members of the TGF-β family may act via ActRIIB, including BMP-4 [Chang et al., 1997] and Vg1 [Schulte-Merker et al., 1994], so the effect of the mutation may be to interfere with signaling by several ligands.
There are important interactions between the notochord and the establishment of LR asymmetry. Several lines of experimental evidence point to the midline as a LR signaling source.
In humans, several combinations of LR abnormalities occur more commonly in heterotaxia syndromes. These syndromes are unlikely to be representative of the full spectrum of embryonic LR abnormalities as many would be expected to be lethal. The more common heterotaxies include: (1) discordance in the LR arrangement of two normally asymmetrical structures (e.g., situs solitus of the thorax with situs inversus of the viscera); (2) isomerism, wherein a normally asymmetric structure, or pair of structures, develops mirror-image symmetry, as in the case of pulmonary isomerism; (3) retention of primitive symmetry in a normally asymmetric structure, as in failure of the gut to rotate. These patterns of heterotaxia are often associated with asplenia or polyspenia.
In asplenia field defect, the lungs are frequently trilobed, both bronchi are eparterial, there are two right atrial appendages and bilateral sinus nodes, the liver is in the midline, and the spleen is absent or diminished. Cardiac anomalies include common atrium, complete AV canal, double-outlet right ventricle, transposition of the great arteries, and anomalous pulmonary venous connections. In polysplenia, the lungs are often bilobed, both bronchi are hyparterial, there are two left atrial appendages, the spleen is multilobed, and the inferior vena cava is interrupted. The associated cardiac anomalies are similar to those of asplenia, but occur at a lower frequency. The lack of LR coordination between adjacent heart segments may account for these problems. Thus, the atria and abdominal organs do not assume their position with regularity, cardiac looping appears randomized, and ventriculoarterial connections are not predictable.
Goldstein et al. [1998], using molecular and experimental embryology as a framework for understanding human laterality defects, have proposed a model to explain the development of various cardiac anomalies. They have proposed that errors of cardiac asymmetry result from one of two abnormalities in LR signaling: (1) the normal LR pathway is reversed, and the embryo develops as a mirror-image reversal (situs inversus totalis); (2) an asymmetric signal is either absent or bilaterally symmetric, or it is normally expressed, but the heart is unable to receive the signal (Fig. 16). The latter leads to a loss of LR identity in the heart. Consequently, each of the anatomic segments chooses sidedness randomly and independently, resulting in a variety of discordant relationships.
Laterality defects were present in the conjoined hearts we have presented. These were associated with asplenia and anomalies of systemic venous return in Case 4, and bilateral polysplenia in Case 5. In the specimen from a D&E splenic tissue was not identified, however, the specimen was incomplete, and therefore it was not adequate to evaluate. These hearts were also characterized by left-to-right abnormalities (heterotaxia) common to the poly/asplenia syndromes.
Acknowledgements
We thank Dr. H. Joseph Yost of the University of Utah for critical review of the manuscript.




