Smoking, folate and methylenetetrahydrofolate reductase status as interactive determinants of adenomatous and hyperplastic polyps of colorectum
Abstract
Most studies demonstrate increased risk of colorectal cancer (CRC) and adenomas in folate-deficient subjects or that high folate intake may afford some protection. Smoking increases such risk in some but not all studies. We investigated whether smoking, folate status and methylenetetrahydrofolate reductase (MTHFR) genotype predict the risk of adenomatous and hyperplastic polyps of colorectum. By colonoscopy, the type, number, size and extent of dysplasia of colorectal polyps were assessed in 443 subjects aged 63–72 years. We also determined RBC folate and the C667T polymorphism of the methylenetetrahydrofolate reductase (MTHFR) gene. Smoking, folate status and the C677T MTHFR polymorphism were strong, interactive determinants of high-risk adenomas (HRAs, defined as adenomas ≥10 mm in diameter, adenomas with villous components or with severe dysplasia). The risk was particularly high in smokers with low folate and the CT/TT genotype (risk category T) and in smokers with high folate and the CC genotype (risk category C). With non-smokers with low folate and the CC genotype as reference, the odds ratios (OR, 95% CI) were 8.7 (2.5–29.7) in category T and 9.9 (2.6–38.4) in category C. Notably, this risk pattern was also observed for hyperplastic polyps. In conclusion, in smokers, high folate status may confer increased or decreased risk for HRAs, depending on the MTHFR genotype. These data demonstrate the strong gene-nutrition interaction involving the C677T MTHFR polymorphism. © 2001 Wiley-Liss, Inc.
INTRODUCTION
Adenomas are a common finding in the colon, and the prevalence increases with age [Vatn and Stalsberg, 1982; Rex et al., 1993]. Sixty to ninety percent of the colorectal cancers (CRC) are believed to develop from adenomas [Morson, 1974; Vogelstein et al., 1988; Atkin et al., 1992]. Knowledge of the factors determining the development of adenomas and their progression to CRC is sparse.
Epidemiological studies bring strong evidence that dietary folate intake protects against CRC and colon adenomas [Kim, 1999]. Long-term use of multivitamins containing folic acid confers a substantial reduction of CRC risk [Giovannucci et al., 1998]. Studies based on measures of folate status in blood, however, provide discrepant results. A negative association between red blood cell (RBC) folate and colonic adenomas has been demonstrated in United States males [Bird et al., 1995], whereas no relation was found in male Finnish smokers [Glynn et al., 1996].
The risk of CRC in relation to the common C677T polymorphisms [Frosst et al., 1995] of the folate metabolizing enzyme, methylenetetrahydrofolate reductase (MTHFR, EC 1.5.1.20) has been studied in United States health professionals [Chen et al., 1996; Ma et al., 1997]. Two studies demonstrate a substantial risk reduction in subjects homozygous for the T-allele as compared with heterozygous CT or homozygous CC individuals. Notably, the protection was abolished in subjects with low methionine intake, high alcohol consumption [Chen et al., 1996] or in folate-deficient subjects [Ma et al., 1997], demonstrating a gene-nutrition interaction. On the other hand, a risk enhancement for colorectal adenomas has recently been found in subjects with low folate status and the T-allele [Ulrich et al., 1999; Levine et al., 2000].
The modulation of cancer risk by the C677T MTHFR polymorphism is conceivable because of the role of the enzyme in folate metabolism [Rozen, 1997]. MTHFR catalyses the irreversible conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. The former folate species is critical in DNA biosynthesis, whereas 5-methyltetrahydrofolate serves as a methyl-donor in homocysteine remethylation to methionine [Finkelstein, 1990]. The T-allele is associated with an enzyme variant characterized by reduced enzyme activity in vitro and impaired homocysteine remethylation under conditions of folate deficiency [Guttormsen et al., 1996; Rozen, 1997]. Thus, the C677T transition affects a regulatory locus directing folates to either methionine or DNA biosynthesis.
There are consistent reports on the association of cigarette smoking with colorectal adenomatous polyps, whereas only recent data convincingly demonstrate that smoking increases the risk for CRC [Giovannucci and Martinez, 1996; Chao et al., 2000; Sturmer et al., 2000]. The studies on CRC demonstrating a strong association between smoking and CRC usually have long-term follow-up and population-based controls [Heineman et al., 1994; Giovannucci and Martinez, 1996]. This may reflect a long induction period for CRC, and that smoking promotes an early stage in carcinogenesis [Hoff et al., 1987].
In the present cross sectional study, we investigated the associations between smoking, folate status and MTHFR genotype with the occurrence of adenomas and hyperplastic polyps in colorectum, detected by colonoscopy of a population-based sample of 63–72 years old individuals.
MATERIALS AND Methods
Subjects and Protocol
The subjects included were participants in Telemark Polyp Study I (TPS-I), which investigated CRC prevention by flexible sigmoidoscopy and polypectomy. TPS-I started in 1983. 799 males and females, born in 1924–33, were drawn from the population registry and randomized into an endoscopic screening and a control group [Hoff et al., 1985]. The primary end-point was CRC.
All subjects enrolled in TPS-1 were in 1996 offered colonoscopy and removal of polyps. Two hundred and ten individuals from the screening group and 241 controls, altogether 71% of the individuals still alive in the two groups, attended.
The present study is a cross sectional analysis of data obtained in 1996 by questionnaire, colonoscopy and biochemical and genetic analysis.
Smoking (current smoker, number of cigarettes per day, former smoker) and the use of NSAIDs were ascertained from a questionnaire.
In both groups, a detailed assessment of polyp status was done by recording the number, type, and size of polyps. Dysplasia was graded as low, moderate and severe, according to the WHO classification [Jass and Sobin, 1989]. Adenomas containing more than 25% villous structures were defined as adenomas with villous components. Adenomas with a diameter ≥10 mm or severe dysplasia or containing villous components were classified as high-risk adenomas (HRAs) [Zarchy and Ershoff, 1994].
The study was approved by the ethical committee at the Haukeland University Hospital.
Blood Collection and Biochemical Analyses
Whole blood was collected into vacutainer tubes, with and without EDTA. The serum fraction was isolated one hour after sampling. Blood for EDTA-plasma was immersed in ice immediately after sampling, and plasma was obtained by centrifugation within 30 min after sampling. Serum and EDTA plasma were stored at −70°C until analysis.
Total plasma homocysteine (tHcy) was analyzed by HPLC and fluorescence detection [Fiskerstrand et al., 1993], serum folate by a microbiological method [Molloy and Scott, 1997], and RBC folate by the Quantaphase folate radioassay produced by Bio-Rad Laboratories (Hercules, CA).
Analysis of MTHFR Genotype
Genotyping was carried out in 1 μl whole blood added directly to the reaction vessel. The blood was overlaid with 50 μl of PCR mastermix and subjected to 33 thermocycles. The allele specific PCR products were analyzed by multiple-injection capillary electrophoresis as described previously [Ulvik et al., 1998].
Statistical Analysis
The values for tHcy, serum folate and RBC folate were markedly skewed, and these variables were therefore logarithmically transformed, and presented as geometric means with 95% confidence intervals. Factorial analysis of variance (factorial ANOVA) was used to examine the difference in tHcy and vitamin levels between genders and in relation to smoking, and the difference in tHcy, serum and RBC folate according to MTHFR genotype. Predictors of tHcy were identified by linear regression on the log-transformed values.
We used logistic regression to obtain odds ratios of colorectal neoplasias according to smoking, folate and MTHFR status, and with adjustment for other variables (age, gender, BMI, use of NSAIDs, and attendance at screening in 1983) known to affect such risk.
Interactions (effect measure modification) by RBC folate level (above vs. below median) were assessed using a likelihood-ratio test for the product term between the binary folate variable and a linear three-level representation of smoking or MTHFR, respectively. With the combination variable of smoking and MTHFR, interaction by folate was tested with a composite likelihood-ratio test of no effect of the three product terms between the binary RBC folate variable and the indicator terms of the MTHFR/smoking combination variable.
The analyses were performed with the statistical packages SPSS (SPSS Inc. Chicago, version 6.1.1) and SAS (The SAS system for Windows, release 6.12). All P-values are two-sided, and values below 0.05 were considered as significant.
RESULTS
Demographics, Genetics and Clinicopathological Diagnoses
The study population included 229 men and 214 women aged 63–72 years. Men were more often current (32.8%) and former smokers (41.0%) than women (20.6 and 14.0%). The overall frequencies of the TT, CT and CC MTHFR genotypes were 7.7, 39.2 and 53.1%, respectively (Table I).
| Characteristics | Value |
|---|---|
| Demographics | |
| Number | 443 |
| Gender, M/F | 229/214 |
| Age, yr, median (range) | 67 (63–72) |
| BMI, kg/m2 | |
| Males, median (range) | 24.8 (15.4–36.3) |
| Females, median (range) | 25.0 (15.5–40.0) |
| Smoking | |
| Current% | 27.1 |
| No. of cigarettes, median (range) | 12.5 (4–25) |
| Former, % | 28.2 |
| Use of NSAIDs, % | 12 |
| Genetics, MTHFR Status, % | |
| CC | 53.1 |
| CT | 39.2 |
| TT | 7.7 |
| Clinicopathological diagnoses | |
| Polyps | |
| No. of subjects with polyps | 327 |
| No. of polyps, median (range) | 3 (1–30) |
| Hyperplastic polyps | |
| No. of subjects with polyps | 215 |
| No. of subjects with polyps n ≥ 3 | 91 |
| No. of polyps, median (range) | 2 (1–30) |
| Adenomas | |
| No. of subjects with adenomas | 177 |
| No. of subjects with adenoma n ≥ 3 | 42 |
| No. of adenomas, median (range) | 2 (1–22) |
| No. of subjects, adenoma diameter ≥ 5 mm | 74 |
| No. of subjects, adenoma diameter ≥ 10 mm | 28 |
| Diameter of largest (mm), median (range) | 4 (1–49) |
| No. of subjects, villous component | 31 |
| Dysplasia, graded 1–4, median (range) | 3 (1–4) |
| No. of subjects, severe dysplasia | 70 |
| No. of subjects, high risk adenomas | 48 |
Colorectal polyps were detected in 327 subjects, hyperplastic polyps in 215 subjects and adenomas in 177 subjects. Variables reflecting numbers, diameter of adenomas and the degree of dysplasia are listed in Table I. Notably, 48 subjects had high-risk adenomas (HRAs).
Blood Indices and MTHFR Status
The geometric mean (5–95th centiles) was 9.1 (5.9–17.2) μmol/L for tHcy, 10.0 (5.5–23.7) nmol/L for serum folate, 263 (142–559) nmol/L for RBC folate and 294 (146–654) pmol/L for serum cobalamin. Both serum and RBC folate were lower in smokers than non-smokers, but only serum folate showed a gender-related difference with higher levels in females than males (Table II). The concentrations of tHcy and serum folate, but not RBC folate, were related to the C677T MTHFR polymorphism, with highest level of tHcy and lowest levels of serum folate in the TT subjects (Table III).
| Parameter | All | Male | Female | Gender (P) | Smoking (P) | ||
|---|---|---|---|---|---|---|---|
| Smoker | Non-smoker | Smoker | Non-smoker | ||||
| n | 442 | 75 | 154 | 45 | 168 | ||
| tHcy | 9.5 | 10.5 | 9.6 | 9.9 | 8.8 | 0.04 | 0.004 |
| (µmol/L) | (9.2–9.8) | (9.8–11.4) | (9.1–10.1) | (8.6–11.3) | (8.4–9.2) | ||
| Serum folate | 10.4 | 8.8 | 10.2 | 10.2 | 11.5 | 0.006 | 0.006 |
| (nmol/L) | (10.0–10.8) | (7.9–9.7) | (9.5–10.9) | (8.5–12.1) | (10.7–12.3) | ||
| RBC folate | 277 | 258 | 296 | 256 | 276 | 0.4 | 0.02 |
| (nmol/L) | (267–289) | (231–288) | (276–318) | (223–293) | (260–293) | ||
| Serum cobalamin | 295 | 275 | 302 | 298 | 297 | 0.6 | 0.4 |
| (pmol/L) | (282–307) | (246–308) | (208–326) | (266–333) | (276–318) | ||
- * Factorial ANOVA, adjusted for age.
- Data are given as geometric mean and 95% CI in parenthesis.
| Parameter | MTHFR Genotype | P* | ||
|---|---|---|---|---|
| CC | CT | TT | ||
| tHcy | 9.1 | 9.6 | 11.5 | <0.001 |
| (µmol/L) | (8.7–9.5) | (9.15–10.0) | (9.6–13.8) | |
| Serum folate | 10.9 | 10.2 | 8.2 | 0.001 |
| (nmol/L) | (10.3–11.6) | (9.5–10.9) | (7.0–9.5) | |
| RBC folate | 277 | 281 | 270 | 0.9 |
| (nmol/L) | (261–293) | (264–298) | (231–314) | |
- * Factorial ANOVA, adjusted for age and gender. The dependent variables are log transformed.
- Data are given as geometric mean and 95% CI in parenthesis.
High-Risk Adenomas
The overall risk for HRAs was significantly (P<0.006) increased at low RBC folate, with an OR (95% CI) of 3.05 (1.34–6.96) comparing the lowest with the highest tertile (Table IV). The other indicators of folate status, like serum folate and tHcy, showed a weaker (serum folate) or no (tHcy) association with risk of HRAs (data not shown).
| All | Low RBC Folate (<263 nmol/L) | High RBC Folate (>263 nmol/L) | P-interact | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRA (N) | Tot (N) | % | OR | 95% CI | P-trend | OR | 95% CI | P-trend | OR | 95% CI | P-trend | ||
| Model I | |||||||||||||
| RBC folate | |||||||||||||
| 320–1186 | 10 | 141 | 7.1 | 1 | |||||||||
| 230–319 | 14 | 162 | 8.6 | 1.30 | (0.54–3.16) | ||||||||
| <230 | 24 | 139 | 17.3 | 3.05 | (1.34–6.96) | 0.006 | |||||||
| MTHFR | |||||||||||||
| CC | 20 | 234 | 8.6 | 1 | 1 | 1 | |||||||
| CT | 21 | 173 | 12.1 | 1.51 | (0.76–2.99) | 3.53 | (1.38–9.05) | 0.31 | (0.10–1.00) | ||||
| TT | 6 | 34 | 17.7 | 2.41 | (0.82–7.06) | 0.08 | 6.06 | (1.43–25.7) | 0.002 | 0.89 | (0.16–4.84) | 0.22 | 0.004 |
| Current Smoking | |||||||||||||
| No | 25 | 323 | 7.7 | 1 | 1 | 1 | |||||||
| 1–9 | 8 | 59 | 13.9 | 1.95 | (0.79–4.81) | 1.74 | (0.54–5.63) | 3.07 | (0.67–14.1) | ||||
| 10–25 | 15 | 61 | 24.6 | 4.00 | (1.86–8.62) | 0.0003 | 3.03 | (1.03–8.86) | 0.04 | 10.20 | (2.95–35.3) | 0.0005 | 0.18 |
| Model II | |||||||||||||
| Smoking/MTHFR | |||||||||||||
| No/CC | 10 | 179 | 5.6 | 1 | 1 | 1 | |||||||
| No/CT or TT | 14 | 142 | 9.9 | 1.77 | (0.75–4.17) | 2.96 | (0.94–9.28) | 0.80 | (0.20–3.17) | ||||
| Yes/CC | 10 | 55 | 18.5 | 3.64 | (1.37–9.62) | 1.54 | (0.34–7.03) | 11.85 | (2.86–49.1) | ||||
| Yes/CT or TT | 13 | 65 | 20.0 | 4.65 | (1.88–11.5) | 0.005a | 8.21 | (2.40–28.1) | 0.006a | 1.99 | (0.47–8.47) | 0.002a | 0.002b |
- * Model I includes above variables plus age, sex, RBC folate, use of NSAIDs (yes/no), flexible sigmoidoscopy in 1983, and BMI (tertiles). Model II includes the same terms except that smoking and MTHFR are represented by a combination variable with four levels (CC-non-smokers, CT/TT-non-smokers, CC-smokers and CT/TT smokers).
- a Test of homogeneity for no effect of the three smoking/MTHFR indicator terms.
- b Likelihood-ratio interaction test of no effect of product terms between RBC folate (<263vs.>263) and the three smoking/MTHFR indicator terms.
MTHFR genotype was analyzed using the whole data set and for RBC folate above and below the median (263 nmol/L). Overall there was no significant association between HRAs and MTHFR status. At RBC folate < 263 nmol/L, the OR of HRA increased significantly (P for trend = 0.002) as a function of the number of T-alleles. Moreover, the CT and TT genotypes both conferred significantly higher risk than the CC genotype (Table IV). Conversely, the CT and TT genotypes showed a weak, although not significant, protective effect at folate above the median (Table IV), and with the CT and TT genotypes combined, there was a significant inverse association (P < 0.05, data not shown). Based on these data, subjects with CT and TT were grouped together in further analyses. The opposite effect of the T-allele at low and high folate, is in agreement with a significant interaction between genotype and RBC folate (P = 0.004, Table IV).
Smoking was a strong risk factor for HRAs. The risk increased in a dose-dependent manner both at low and high RBC folate, with the strongest association (P = 0.0005) at high folate (Table IV).
We constructed a combination variable of smoking and genotype and tested for an interaction between this variable and folate status. A highly significant (P = 0.002) interaction was demonstrated (Table IV).
To compare risk across all strata of folate status, genotype and smoking, we recalculated ORs for all eight categories with non-smokers, low folate and CC genotype as a common reference category. The most notable finding was that smoking is associated with a remarkably high-risk in subjects with the CT/TT genotype and low folate (denoted high-risk category T; OR 8.7 (2.5–29.7)), and in subjects with the CC and high folate (high-risk category C; OR 9.9 (2.6–38.4)). The CT/TT genotype conferred only a moderately increased risk in non-smokers at low folate (Fig. 1). The 95% CI for the ORs of the other groups were wide and included one (Fig. 1).
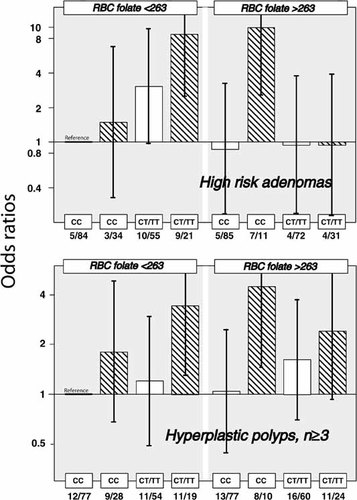
Odds ratio (OR with 95% CI) for HRA or hyperplastic polyps according to genotype, folate status and smoking ORs were calculated in eight categories defined by different combinations of blood folate below and above 263 nmol/L, CC vs CT/TT genotype and smoking. The model adjusts for age, gender, attendance at flexible sigmoidoscopy screening in 1983, BMI, and using NSAIDs. ORs for smokers are given as cross-hatched columns, and ORs for non-smokers as open columns.
Risk of Hyperplastic Polyps
The risk of hyperplastic polyps was investigated in the same eight categories defined by RBC folate, genotype and smoking. Interestingly, the two high-risk categories T and C associated with HRAs (Table IV, Fig. 1) had also significantly increased risk of hyperplastic polyps (number of polyps ≥3, Fig. 1; data not shown for number of polyps ≥1). The other 5 subgroups had ORs in the range 0.7–2 with 95% CI including one (Fig. 1).
DISCUSSION
Study Population and Design
This is a cross sectional study of 443 subjects undergoing screening colonoscopy involving a detailed assessment of type, size, number and the degree of dysplasia of colorectal polyps. Because of the detailed assessment of polyp status, misclassification of colorectal pathology is minimal, which may explain the strong relations reported. In addition, the study population is homogenous with respect to age (range 63–72 years) and ethnicity (Table I). Finally, the subjects enrolled have no clinical overt cancer, and the blood parameters are therefore not influenced by metabolic alterations related to cancer cachexia.
Folate and MTHFR Status
tHcy was higher and both serum and RBC folate levels were lower in smokers than in non-smokers (Table II), which is in agreement with findings consistently reported by others [Witter et al., 1982; Ortega et al., 1994; Piyathilake et al., 1994; Mansoor et al., 1995; Nygård et al., 1995]. Impaired folate status in smokers has been explained by inadequate diet [Berger and Wynder, 1994], and by interference of smoking with folate absorption, distribution [Piyathilake et al., 1994] or metabolism [Abu Khaled et al., 1986].
The frequency of the C677T MTHFR variants (7.7, 39.2 and 53.1% of the TT, CT and CC genotypes, respectively; Table I) is similar to the distribution of genotypes reported for other Caucasian populations [Gudnason et al., 1998; Pepe et al., 1998]. We also confirmed [Brattstrom et al., 1998; Gudnason et al., 1998] higher tHcy and lower serum folate in homozygous TT subjects as compared with the other C677T MTHFR variants (Table III).
Association of HRAs With Folate and MTHFR Status
Adenomas were categorized as HRAs according to Zarchy and Ershoff [1994], and were studied in detail because they carry a particularly high risk of malignant transformation [Shinya and Wolff, 1979; Vogelstein et al., 1988; Atkin et al., 1992].
RBC folate was inversely associated in a dose-dependent manner with HRAs (Table IV). Serum folate showed a similar, but weaker association, whereas no association was found with the folate marker, tHcy. The latter observation seems to be in conflict with a recent report on indicators of folate status in colonic mucosa [Kim et al., 1998], but may be related to the observations that tHcy is influenced by renal function in this age group [Brattstrom et al., 1994]. Furthermore, tHcy increases markedly only at deficient folate levels [Rasmussen et al., 1996; Brouwer et al., 1998] whereas modulation of risk is observed over the whole folate spectrum (Table IV), as has also been suggested by others [Lashner, 1993; Mason and Levesque, 1996].
Chen et al. [1998] recently reported on no association of the MTHFR TT genotype with colorectal adenomas and no interaction between the MTHFR polymorphism and folate intake. In contrast, we observed strong interaction between folate status and genotype (P = 0.005, Table IV). At high folate, there was a weak inverse association between the number of T-alleles and HRAs, whereas there was a strong positive association at low folate. Our data therefore are in agreement with the results from two recent studies on CRC demonstrating a protective role for the TT genotype [Chen et al., 1996; Ma et al., 1997] that was abolished by alcohol consumption or folate deficiency [Chen et al., 1996]. A lower intake of folate in our study population of elderly Norwegians than among United States health professionals is likely, and may explain the different impact of the CT/TT genotype.
Two Categories With High Risk for Both Colorectal Adenomas and Hyperplastic Polyps
Strong interactions were observed between folate status and both MTHFR genotype (P = 0.004; Table IV) and the combined smoking-MTHFR variable (P = 0.002; Table IV, Fig. 1). We observed that smoking conferred a high-risk of HRAs in subjects of the CT/TT genotypes at low folate. The most striking and unexpected finding was an equally high-risk of HRAs in the subgroups with the CC genotype at high folate. Notably, this risk pattern was also observed for hyperplastic polyps (Fig. 1). Because the prevalence of hyperplastic polyps (n = 215; n = 91 for ≥ 3 polyps) was much higher than that of HRAs (n = 48) (Table I), this observation adds credence to the concept of two risk categories. We denoted these two groups high-risk categories T and C.
Implications
Our findings regarding the interaction between folate status, MTHFR genotype and smoking may explain some apparently conflicting published results. Low RBC folate has been reported in United States patients (20.5–39.4% smokers) with colorectal adenomatous polyps [Bird et al., 1995]. This contrasts to no relation between blood folate and CRC in Finnish smokers [Glynn et al., 1996]. Actually, in the latter study, a 2-fold increase in rectal cancer risk was found for men with serum folate < 2.9 ng/ml and those in the highest quartile of energy-adjusted folate intake [Glynn et al., 1996]. We observed no association between RBC folate and HRAs when smokers were analyzed separately (data not shown). Thus, inconsistent results on the relation between colorectal neoplasia and folate status [Chen et al., 1996, 1998; Ma et al., 1997; Slattery et al., 1999] may reflect variable prevalence of former and current smokers in different study populations.
Possible Mechanisms
Smoking probably affect an early stage in carcinogenesis, mediated by a variety of carcinogens present in tobacco smoke [Potter, 1999] that enhances the development of early lesions like hyperplastic polyps and adenomas. Mechanisms for the modulation of carcinogenesis by folate, include DNA hypomethylation, uracil mis-incorporation into DNA and disruption of DNA repair [Mason and Levesque, 1996; Kim et al., 1998]. Because folate status is associated with CRC as well as adenomas [Glynn et al., 1996; Chen et al., 1999], folate may modulate early and late stages of the progression toward cancer. Low folate status may cause DNA hypomethylation [Choi and Mason, 2000; Piyathilake et al., 2000; Rampersaud et al., 2000], possibly caused by elevated S-adenosylhomocysteine [Yi et al., 2000]. The effect may be further enhanced by low MTHFR activity associated with the T-allele [Stern et al., 2000].
The low MTHFR activity associated with the T-allele may cause low methionine and thereby DNA hypomethylation at low folate. In contrast, the combination of smoking and high folate status in CC subjects probably promotes the development of colorectal polyps by a different mechanism(s), and may reflect the cancer “acceleration phenomenon,” that has been substantiated by both clinical and experimental data [Kim, 1999]. These possible mechanisms are depicted in Figure 2.
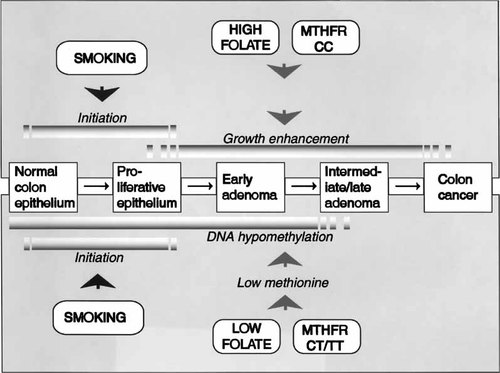
Hypothetical model for the involvement of folate and MTHFR status in the pathogenesis of colorectal adenoma and cancer in smokers This simplified model focuses on the pathways that may explain the high risk for HRAs in subject categories C and T, and does not include the mechanisms (uracil mis-incorporation and impaired purine and pyrimidine synthesis) that may contribute to the overall risk enhancement associated with folate deficiency in non-smokers.
CONCLUSION
Folate status and the C677T MTHFR polymorphism profoundly modulate the procarcinogenic effect of smoking on the colorectal mucosa. Particularly, HRAs with a high malignant potential, were observed in smokers with either low folate and the CT/TT genotypes, or with the combination of high folate and the CC genotype. Similar, but weaker relations were observed with hyperplastic polyps, which are not regarded as precursors of cancer [Provenzale et al., 1990; Waye and Bilotta, 1990], but may serve as an indicator of populations at risk [Kearney et al., 1995]. These findings may explain some inconsistent data on folate status and CRC, but also add a cautionary note to the debate on folate fortification [Anonymous, 1996].
Acknowledgements
The work was supported by grants from the Norwegian Cancer Society, Arve Ulvik is a research fellow of the Norwegian Cancer Society.




