Novel Igα (CD79a) gene mutation in a Turkish patient with B cell-deficient agammaglobulinemia
Abstract
Mutations that impair early B cell development result in profound antibody deficiency, which is characterized by a paucity of mature B cells and the early onset of recurrent pyogenic infections. Among these inherited early B cell defects, X-linked agammaglobulinemia (XLA) with mutations in Bruton's tyrosine kinase (BTK) gene is mostly identified. Recent studies have shown that mutations in the gene for μ heavy chain (IGHM) and for other components of the pre-B cell receptor complex, including λ5/14.1 (IGLL1) or Igα (CD79a), can cause a disorder that is clinically similar to XLA. In a genetic survey of XLA in Turkey, we examined possible mutations in the IGHM, IGLL1, and Igα genes in some male patients with presumed XLA who did not have identifiable BTK mutations. We found an eight-year-old boy with a novel homozygous mutation in the Igα gene (IVS2+1G>A) causing B cell defect. This is the second case of agammaglobulinemia due to an Igα (CD79a) deficiency in the world. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Genetic defects in early B cell development cause marked antibody deficiency, which is characterized by early onset recurrent bacterial infections, profound hypogammaglobulinemia, and a lack of circulating mature B cells [Gaspar and Conley, 2000]. A common form of inherited antibody deficiency due to B cell defect is X-linked agammaglobulinemia (XLA). XLA is caused by mutations in Bruton's tyrosine kinase (BTK) gene, which encodes a cytoplasmic tyrosine kinase involved in B cell signal transduction [Tsukada et al., 1993; Vetrie et al., 1993]. Mutations in BTK result in a maturation block from pro-B cells to pre-B cells in the B cell differentiation pathway [Nomura et al., 2000]. Although most male patients with presumed XLA have mutations in BTK, some hypogammaglobulinemic patients, including females, display a clinical phenotype resembling XLA, regardless of the absence of identifiable BTK mutations [Conley et al., 1998]. Since some of non-XLA patients have traceable pedigrees, it has been suggested that non-XLA in such patients may be autosomally inherited [Conley and Sweinberg, 1992]. A number of candidate genes contributing to early B cell development have been searched for mutations in non-XLA patients. Finally, mutations in the gene for μ heavy chain (IGHM), and for some components of the pre-B cell receptor (BCR) complex, including λ5/14.1 (IGLL1) and Igα (CD79a), have been shown to cause autosomal recessive agammaglobulinemia [Yel et al., 1996; Minegishi et al., 1998, 1999a].
In another study, we attempted to identify BTK mutations in Turkish males with presumed XLA. In addition, efforts were made to detect possible mutations in IGHM, IGLL1, or Igα genes in non-XLA patients. As a result, we found a boy with a novel homozygous mutation in the Igα gene causing B cell defect. Herein we describe this patient, the second case of an Igα (CD79a) deficiency in the world.
CLINICAL REPORT
The patient with Igα (CD79a) deficiency was an eight-year-old Turkish boy, who was born to nonconsanguineous healthy parents after uneventful pregnancy and delivery. Recurrent lower respiratory infections and otitis media were evident around age eight months. At age 13 months, he was referred to Hacettepe University Children's Hospital because of progressive muscular weakness without any focal neurologic signs. At this time, his serum immunoglobulin (Ig) levels were markedly low [IgG, 52 mg/dl (normal value, 762 ± 209 mg/dl); IgA, < 21 mg/dl (58 ± 23 mg/dl); and IgM 13 mg/dl (50 ± 24 mg/dl)]. Analysis of peripheral blood lymphocytes revealed that he had fewer than 0.1% CD19+ B cells (normal value, 5.5 ± 4.1%), but normal numbers of T cells [CD3, 88% (65.3 ± 6.7%)]. Muscular weakness improved gradually within three months after initiation of intravenous γ-globulin therapy. He did relatively well with occasional upper and lower respiratory tract infections but developed thickening of the skin and subcutaneous tissue suggestive for dermatomyositis after age two years. Intravenous γ-globulin could not be given regularly until age five years and dermatomyositis-like symptoms became marked when he stopped γ-globulin therapy for four months. After resumption of γ-globulin replacement therapy, his skin and muscle involvement improved but did not heal completely. He had been doing relatively well for the last two years again with occasional respiratory tract infections and diarrhea. However, dermatomyositis-like symptoms and signs continued, and he died of pulmonary infection. The family history showed that his two brothers died of pulmonary infections at age eight months (Fig. 1A). Thus, he was suspected having XLA, and subjected to a mutation analysis in BTK. However, no mutations in the coding and promoter regions in BTK could be detected in his sample.
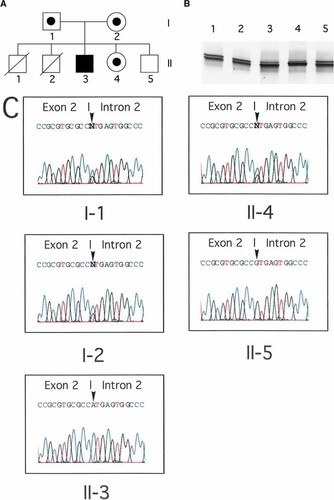
Identification of a mutation in the Igα gene. A: Pedigree of the patient's family. His elder brothers died of infections in early infancy. B: PCR-SSCA of exon 2 of the Igα gene from non-XLA patients and normal controls. Abnormal mobility was demonstrated in lane 4 (the patient). Lanes 1, 2, and 3 indicate other non-XLA patients, and lane 5 indicates a normal control. C: Electropherograms of exon 2 of the Igα gene from the patient and his family members. They showed a substitution of G with A at the invariant +1 position of the splice donor site for exon 2 in the patient. The mutation sites are noted by arrowheads.
To examine the possibility of autosomal recessive B cell defects, his genomic DNA was analyzed for mutations in IGHM, IGLL1, or Igα genes by polymerase chain reaction (PCR) and single strand conformation polymorphism analysis (SSCA) as described previously [Yel et al., 1996; Minegishi et al., 1998, 1999a]. While mutations were identified in neither the IGHM nor the IGLL1 genes, PCR-SSCA demonstrated an abnormal electrophoretic mobility regarding exon 2 of the Igα gene (Fig. 1B). Direct sequencing of the Igα gene exon 2 from the patient revealed a homozygous alteration (G to A) at an invariant splice donor site of intron 2 (IVS2 +1) (Fig. 1C). This altered nucleotide was not detected in 60 healthy Turkish persons, indicating that it was not a polymorphism. The patient's father, mother, and sister were all G/A heterozygotes at the IVS2 +1 site (Fig. 1C). His living brother had a normal sequence of the Igα gene exon 2. Although cDNA from the patient was not available, the homozygous alteration at the splice donor site may have caused skipping of exon 2, presumably resulting in the truncation of Igα upstream of the transmembrane domain.
DISCUSSION
We identified an eight-year-old Turkish boy with a homozygous splice defect possibly resulting in the truncation of Igα upstream of the transmembrane domain. This is the second case of Igα deficiency in the world. His mutation was distinctive from that in the first case of Igα deficiency, who had a homozygous mutation with a single-base substitution at an invariant position of the splice acceptor site for exon 3 of Igα gene [Minegishi et al., 1999a]. Intriguingly, the first case of Igα deficiency was also Turkish. Although this patient was born to parents without known consanguinity, his parents were from the same small village. It was noted that both cases had homozygous mutations, seemingly reflecting a high rate of consanguineous marriages in Turkey [Tuncbilek and Koc, 1994].
Igα (CD79a) is an invariant component of the pre-BCR and BCR complex [Karasuyama et al., 1997]. In the process of B cell development, the B cell precursors transiently express a pre-BCR complex, which consists of a rearranged μ heavy chain, surrogate light chain λ5/14.1, and VpreB, Igα and Igβ. Mutation in any genes associated with pre-BCR complex possibly result in an arrest of B-cell development. Non-XLA patients with B cell defect have been demonstrated to have mutations in IGHM, IGLL1, or the Igα genes [Yel et al., 1996; Minegishi et al., 1998, 1999a]. Immunofluorescence analysis of the bone marrow from the first case with an Igα gene mutation have indicated that Igα deficiency may cause a complete block in early B cell development from pro-B to pre-B transition as seen in μ heavy chain-deficient patients [Minegishi et al., 1999a]. Although the bone marrow cells were not examined in our case, the detection of a novel Igα gene mutation seems to validate that Igα plays a critical role in the development of normal B cells.
A patient with non-XLA hypogammaglobulinemia has been reported to have a compound heterozygous defect in the cytoplasmic adapter protein BLNK [Minegishi et al., 1999b]. It is likely that deficiencies in certain molecules, which are involved for pre-BCR-mediated signal transduction in early B cell development, may cause antibody deficiencies lacking mature B cells as well. Similarly to human XLA, mutation in the Btk gene is responsible for the murine model of X-linked immunodeficiency (xid) [Rawlings et al., 1993]. Recently, a knockout maneuver has demonstrated that mice deficient in protein kinase Cβ [Leitges et al., 1996], p85α subunit of phosphoinositide 3-kinase [Fruman et al., 1999; Suzuki et al., 1999], and phospholipase Cγ2 [Wang et al., 2000] can result in B cell deficiency resembling the xid mice. Mutations in these molecules may be candidates for human B cell defects, since mutations in IGHM, IGLL1, Igα, and BLNK have not accounted for all of human non-XLA hypogammaglobulinemia [Conley et al., 2000].
Acknowledgements
We appreciate the generous participation of the patient and his family members in the present study.




