Anticipation in a unique family with Charcot-Marie-Tooth syndrome and deafness: Delineation of the clinical features and review of the literature
Abstract
Charcot-Marie-Tooth disease (CMT) is a clinically and genetically heterogeneous group of polyneuropathies characterized by degeneration of peripheral nerves, resulting in distal muscle atrophy, sensory loss, and deformities of hands and feet. We have studied 34 individuals in a large 84-member four-generation central Illinois family with autosomal dominant Charcot-Marie-Tooth and deafness. Nerve conduction velocities are consistent with type 1 CMT. Audiological evaluation revealed both auditory neuropathy and cochlear involvement in affected individuals. There is increasing clinical severity and younger age of onset of CMT and deafness with each progressive generation, suggestive of anticipation (P < 0.05). The proband, a female diagnosed at birth with hypotonia, bilateral vocal cord palsy, swallowing incoordination, and hearing impairment, died at age 18 months. Another individual died at the age of 3 months from hypotonia later attributed to CMT. Genetic analysis indicated that affected individuals in this family do not have the common 1.4 Mb duplication associated with type 1A CMT; however, all affected individuals have a unique G to C transversion at position 248 in coding exon 3 of the peripheral myelin PMP22 gene located on chromosome 17p11.2-p12. This mutation is predicted to cause an Ala67Pro substitution in the second transmembrane domain of PMP22, consistent with the molecular cause of the CMT phenotype. However, it does not explain the cochlear component of the deafness, the clinical observation of anticipation, and other features in this family. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Charcot-Marie-Tooth disease (CMT) comprises a clinically and genetically heterogeneous group of polyneuropathies affecting about 1 in 2,500 individuals [Skre, 1974]. A degenerative process of peripheral nerves, CMT results in distal muscle atrophy of the hands and feet, peroneal atrophy, and equinovarus deformity of the feet.
The family of autosomal dominant CMT can be classified into two major categories, CMT types 1 and 2 [Lupski et al., 1991; Dyck et al., 1992]. CMT type 1 (CMT1) displays uniformly decreased nerve conduction velocity (< 38 m/s) associated with a demyelinating hypertrophic neuropathy. CMT disease type 1A (CMT1A, OMIM 118220) is the most common form and links to chromosome 17p11.2p12. Duplication of a 1.5-Mb region on chromosome 17p is associated with CMT1A in the majority of cases. Overexpression of a gene for CMT1A, peripheral myelin protein-22 (PMP22), which maps within the duplication and encodes a myelin-specific protein, has been implicated in the majority of individuals with this disorder [Roa and Lupski, 1993]. CMT type 1B is linked with Duffy blood group on 1q21.3-q23 and is associated with point mutations in the myelin protein zero (P0) gene (OMIM 118200).
CMT type 2 is pathologically and genetically distinct from CMT type 1. Nerve conduction is normal but neuronal degeneration occurs. Linkage analysis in six large autosomal dominant CMT2 (type 2A, OMIM 118210) families demonstrated linkage to a series of microsatellite markers in the distal region of the short arm of chromosome 1p36 [Ben Othmane et al., 1993]. CMT type 2B (OMIM 600882) has been mapped to chromosome 3q13-q22 [Kwon et al., 1995].
CMT with deafness is unusual but has been described in some autosomal dominant families. De Weerdt and Heerspink [1974] reported autosomal dominant neuropathy and hearing loss in a large family. Hearing loss onset began in the 30–40s and was slowly progressive. Young and Harper [1980] reported a large kindred with similar neurologic abnormalities (individuals, however, had normal sensation and normal nerve conduction velocities) and vocal cord paralysis but with normal hearing. Boltshauser [1989] described motor-sensory neuropathy, vocal cord paralysis, and sensorineural hearing loss in three individuals in a large three-generation family. Hamiel et al. [1993] reported a family from Israel with CMT and sensorineural deafness, features which were apparent in infancy and early childhood. Anticipation was noted in this family, with the onset of symptoms occurring earlier with each generation. Hamiel et al. excluded the duplication associated with CMT type 1A. The locus for CMT type 1B on chromosome 1q21-q23 was also excluded through phenotyping of the Duffy blood group.
Our study involves a large central Illinois family originally reported by Kouseff et al. [1982] (OMIM 118300) with autosomal dominant CMT and deafness. Onset occurs in childhood with weakness of the peroneal muscles, followed by atrophy, pes calcaneovarus, steppage gait, poor balance, diminished sensation in the legs, hammer toes, pes cavus, claw hands, and absent deep tendon reflexes. The authors, however, did not report the loss of laryngeal function, electrophysiologic analysis of nerve conduction velocity, or clinical evidence of anticipation. We have previously published the linkage and mutation analysis in this family indicating that the disease is associated with a unique G to C transversion at position 248 in coding exon 3 predicted to cause an Ala67Pro substitution in the second transmembrane domain of the peripheral myelin PMP22 protein [Kovach et al., 1999]. This report expands the clinical phenotype of CMT caused by a unique point mutation in the PMP22 gene.
METHODS
Clinical Studies
Approval for this study was obtained from the Springfield Committee for Research in Human Subjects (SCRIHS protocol #97-060). We enrolled 84 members of a large central Illinois family with autosomal dominant CMT and deafness. This group includes 34 affected individuals, the remaining individuals representing unaffected individuals and spouses. Evaluation of the pedigree (Fig. 1) indicates an autosomal dominant type of inheritance. History of CMT, hearing impairment, and hoarseness was elicited, a general clinical and neurological examination was performed, and nerve conduction studies performed by standard methods.
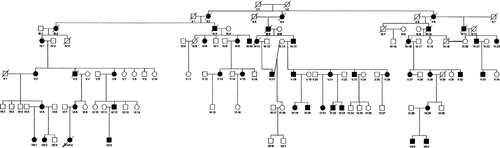
Pedigree of the family with Charcot-Marie-Tooth and deafness. Males are denoted by squares and females by circles. The filled squares and circles indicate affected individuals; unaffected individuals and spouses are indicated by open squares and circles. A diagonal line indicates deceased individuals. The arrow indicates the proband or index case. Numbers I through VII indicate the oldest to the youngest generation in this study.
Audiological Assessment
The majority of individuals had routine audiology studies and 20 had comprehensive audiologic assessments, including otoacoustic emissions (OAEs), sensitive measures of cochlear function, and auditory brainstem response (ABR), a sensitive measure of the function of the VIIIth nerve and auditory brainstem pathways through the level of the lateral lemniscus. OAEs and ABR testing can also sometimes reveal abnormalities in the auditory pathway before they are readily apparent clinically.
Anticipation
From the clinical data, there was a suggestion of increasing clinical severity and younger age of onset of the neurological features and deafness with each progressive generation, highly suggestive of anticipation. The documented or reported mean age of onset of features of CMT and deafness in different generations was analyzed statistically using analysis of variance (ANOVA). If anticipation is biased by earlier diagnosis, then symptom severity will be milder when first assessed. If measures that are biased both positively (age at onset) and negatively (symptom severity) for ascertainment show anticipation, it will be concluded that anticipation, not ascertainment bias, is present.
Molecular Analyses
DNA extraction from archival tissue
Autopsy material provided the source of DNA used in genetic analysis for individual VI:22. Specifically, genomic DNA was extracted from 4 μ histology slides of the patient's liver. A modified extraction protocol was developed, using a combination of published tissue extraction protocols [Diaz-Cano et al., 1997; Howe et al., 1997; Weirich et al., 1997]. Briefly, tissue sections were deparaffinized with two 10-min extractions in fresh xylene. The samples were then dehydrated in serial washes of 100% ethanol, 10 min each, and allowed to dry. The cells were collected from the slides with 200 μL of proteinase K digestion buffer (10 mM Tris-HCl (pH 7.8), 5 mM EDTA, 0.5% SDS) containing 8 μL of proteinase K (10 mg/mL). Proteinase K digestion proceeded for 5 days at 55–65°C, during which 8 μL of fresh proteinase K was added daily. At the end of the digestion period, the proteinase K was inactivated by heat denaturation at 94°C for 15 min. At this time, soluble protein was precipitated by the addition of 50 μL saturated NaCl solution. The sample was vortexed for 5 min, followed by centrifugation at 4,000 rpm for 15 min at room temperature. The DNA fraction contained in the supernatant was precipitated with ethanol using 10 μg yeast tRNA as carrier nucleic acid followed by resuspension in a final volume of 75 μl water.
Repeat expansion detection (RED) assay
A modification of the RED protocol [Schalling et al., 1993; Sirugo and Kidd, 1995; Hofferbert et al., 1997] was employed to analyze expansion of triplet repeats. Reactions consisted of 5 μg of genomic DNA and 10 pmol of phosphorylated, PAGE-purified oligonucleotide (CTG10, CGG10, or GAA10) (Cruachem, Glasgow, Scotland) diluted in 20 μl 1X reaction buffer. Following the addition of Ampligase (5 units) (Epicenter Technologies, Madison, WI), the reactions were cycled 400 times according to the following parameters: for CTG and CGG reactions, 30 sec at 70°C, 10 sec at 95°C; for CTT reaction, 30 sec at 60°C, 10 sec at 95°C. Reaction products were visualized by autoradiography of Southern blot, as previously described [Schalling et al., 1993; Sirugo and Kidd, 1995; Hofferbert et al., 1997].
A more sensitive assay is to investigate length polymorphisms of specific repeat regions, given that the sequence flanking the region of interest is known. Towards this end, the entire 40 Kb genomic coding and noncoding region of PMP22 was scanned for a variety of repeat family sequences. Of particular interest was a (CAG)n triplet repeat element identified in the third intron of PMP22 [Inoue et al., 2001]. In addition, two imperfect dinucleotide repeats are present in intron 3. Simple sequence repeats were also identified in the promoter regions of exon 1A and 1B. Standard PCR with region specific primers was employed to analyze these repeats for polymorphisms (i.e., expansions of rearrangements) that correlate with clinical anticipation.
RESULTS
Clinical Studies
Onset of CMT in affected individuals is evident at a young age by the frequent tripping and delay in achieving milestones. Significant equinovarus deformity of the feet, pes cavus, “foot drop” and abnormal gait develops in the first decade, which in addition to sensory loss causes balance problems. Peroneal muscle atrophy in addition to sensory loss causes balance problems. Atrophy and contractures of the hands also develops, causing clawing and an inability to oppose the thumb with the other digits, often requiring opponensplasty. The disorder is progressive, with individuals requiring a cane or walker for mobility and some becoming wheelchair-bound. Eleven individuals have had lengthening of the Achilles tendon and seven have had triple arthrodesis for ankle stabilization.
Generation IV
Six living individuals in the oldest generation (IV in the pedigree, Fig. 1) ranged in age from 59–69 years (mean 64.2 years). Age of onset of symptoms of CMT in this group ranged from 6–20 years (mean 18.6 years) and all individuals had deafness ranging from ages 20–65 years (mean 46.3 years). A male age 62 years worked as an automobile mechanic with minimal problems noted in his 50s. One 63-year-old developed problems related to CMT in his 30s. A 65-year-old female still lives independently and only wears leg braces. A 59-year-old female was misdiagnosed at the age of 6 years until the correct diagnosis of CMT was made at the age of 14.5 years. She has required hand tendon transfer surgery and despite significant disability is still ambulatory.
Generation V
The 12 individuals studied in generation V range in age from 29–64 years (mean 43.8 years). There is great clinical variation in this group, with age of onset of features of CMT ranging from 5–35 years (mean 11.3 years). Five members of the family have had triple arthrodesis performed in their first or second decade of life. Ages of onset for deafness ascertained in all individuals except in a 33-year-old male ranged from 11–44 years (mean 21.6 years).
Generation VI
Nine living individuals in generation VI range in age from 2–33 years (mean age 15.8 years), with age of onset of the CMT features reported from 2–8.5 years (mean 3.6 years). Hearing loss was reported in six individuals at a mean age of 12.5 years (range 7–32 years).
Deceased individual VI:22 was born following a normal pregnancy and delivery, birthweight 3,487 g. Hypotonia was present at birth. He continued to have muscular weakness and feeding difficulties and died at the age of 3 months in 1984 from an unknown cause. Upon review of his medical records, CMT was suspected in him in view of the severe manifestations in the proband. Haplotype analyses with microsatellite repeat markers and mutation analysis on DNA extracted from histology slides (from autopsy performed on him) indicated that he inherited his mother's disease allele.
Generation VII
The age of onset in the six individuals in generation VII ranged in age from birth to 16 years (mean 10 years). This group includes proband VII:4, who was diagnosed at birth with bilateral vocal cord palsy, swallowing incoordination, and hearing impairment. The respiratory distress was managed with a tracheostomy. Swallowing coordination was managed by gastrostomy tube placement at age 3 weeks. Neurological evaluation revealed hypotonia and decreased tendon reflexes. Brain MRI was normal. ABRs showed prolonged latencies, consistent with auditory neuropathy, although ABR thresholds were normal. Otoacoustic emissions were normal, suggesting normal cochlear function. Nerve conduction studies indicated slow mean conduction velocity of 17 m/s. Subsequently, the baby made excellent progress and achieved milestones. However, at the age of 18 months she died from respiratory problems related to the tracheostomy. The proband's mother, VI:8, age 17 years, was diagnosed with CMT at age 10 years. She was noted to have weakness and muscular wasting of her extremities and hip dysplasia. Electromyography showed evidence of denervation, more marked in distal muscles. She also developed hearing difficulties in her teens. The baby's maternal grandfather, who is now deceased, developed weakness and hearing difficulties in his 20s.
Another individual, a 7-year-old male (VII:5), was also diagnosed at birth and has moderately severe CMT. Two brothers ages 10 and 12 years (VII:8 and VII:9) are cognitively impaired and have attention deficit hyperactivity disorder. Two half-sisters ages 14 and 16 years also have varied manifestation of CMT. The 16-year-old (VII:1) did not develop features of CMT until approximately age 13, however, her younger sister (VII:2) was suspected to have CMT at birth because of nystagmus. Neurological evaluation in her reveals horizontal nystagmus, poor balance, and facial paresis.
Nerve Conduction Studies (Table I)
Clinically affected individuals have nerve conduction velocities < 38 m/s, this level being considered the threshold for differentiating the two autosomal dominant types of CMT. Unaffected individuals all had nerve conduction velocities > 38 m/s, generally > 50 m/s (data not shown). The nerve conduction velocities were compared in different generations and the differences were considered suggestive of anticipation (Fig. 4).
Audiology Testing
Hearing losses ranged from normal hearing thresholds in some children to profound hearing loss in adults. Asymmetric hearing was common, as is typical in auditory neuropathy patients. Of the five children and adolescents included in the study (ages 8–14), one had bilaterally normal pure-tone thresholds, one had normal thresholds in the better ear and borderline normal thresholds in the poorer ear, one had borderline normal thresholds bilaterally, and two had bilateral mild hearing loss. None of the adults had normal or borderline normal hearing in either ear. Two adults had mild hearing loss in the better ear but moderate or moderately severe hearing loss in the poorer ear. Three adults had moderate hearing loss bilaterally and three had moderate hearing loss in the better ear, with moderately severe or severe hearing loss in the poorer ear. Three adults had moderately severe hearing loss bilaterally and one had moderately severe hearing loss in the better ear and profound hearing loss in the poorer ear. Two adults had profound hearing loss bilaterally.
A variety of abnormalities, consistent with auditory neuropathy, were noted on the auditory electrophysiologic test battery for all affected family members. Acoustic reflex abnormalities were also present. The results of the otoacoustic emissions testing, sensitive measures specifically of cochlear function, also suggested cochlear involvement. The patients report that their hearing losses are progressive and hearing aids were typically not helpful.
In eight affected individuals with sensorineural hearing loss, OAE testing suggested cochlear involvement but not of a degree that could fully account for the degree of hearing loss. In others, OAEs were absent. In all affected individuals delayed or absent ABR suggested auditory neural dysfunction. Therefore, this family has both cochlear and neural components to their auditory disorder. Interestingly, the age of onset of peripheral neurologic involvement does not correlate with auditory findings.
We cannot fully rule out the role of recent advances in audiologic assessment methods in detecting hearing loss at an earlier age. For example, the auditory brainstem response was not in widespread clinical use until the 1980s and otoacoustic emissions until the 1990s. However, the family members are convinced that hearing difficulties are apparent earlier in successive generations. Only longitudinal, long-term studies can truly resolve that issue.
Electron Microscopy
A sural nerve biopsy was obtained from the mother of the proband at the time of a tendon transfer operation for foot drop. Light microscopy showed severe loss of myelinated axons of all sizes. Multiple onion bulbs surrounding myelinated and unmyelinated fibers were seen. Electron microscopy studies of the biopsy material showed unmyelinated and some thinly myelinated axons with well-developed onion bulb (OB) formation with remnants of Schwann cell processes (Fig. 5). Denervated bands of Bungner originating from unmyelinated axons are compatible with unmyelinated axonal degeneration. No obvious axonal regeneration clusters were seen.
The parents did not want an autopsy on the proband and nerve pathology was not performed at autopsy done on VI:22, in whom the diagnosis of CMT was made 16 years later.
Anticipation
The mean age of onset of symptoms of CMT in different generations was 18.6, 11.3, 3.6, and 4.4 years, respectively, for generations IV, V, VI, and VII (Fig. 2). The difference was statistically significant (P < 0.05). The mean ages reported for onset of deafness in the three generations IV, V, and VI were 46.3 years, 21.6 years, and 12.7 years, respectively. The difference between the generations was significant (P < 0.001) (Fig. 3). Because of the small numbers, generation VII was not included in the latter analysis. Although we do see the infants and young children from this family clinically, they were not included in this study because they could not provide all the information for the audiologic database.
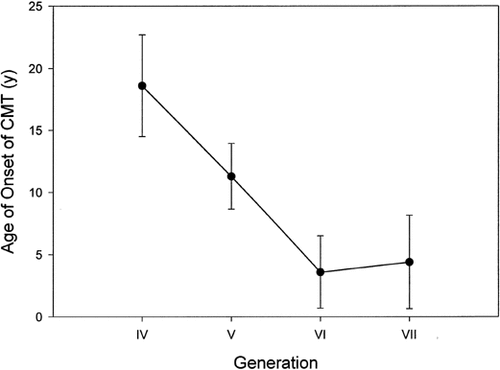
Graph showing lowering of the mean age of diagnosis of CMT in each successive younger generation.
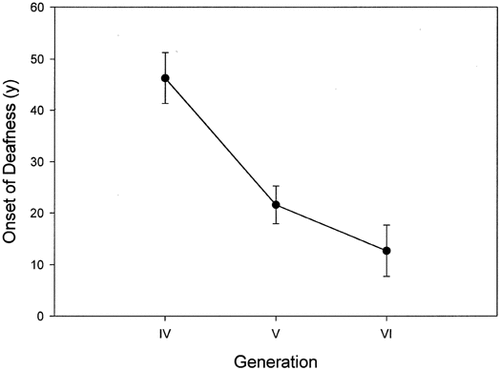
Graph representing the mean age of diagnosis of deafness in different generations.
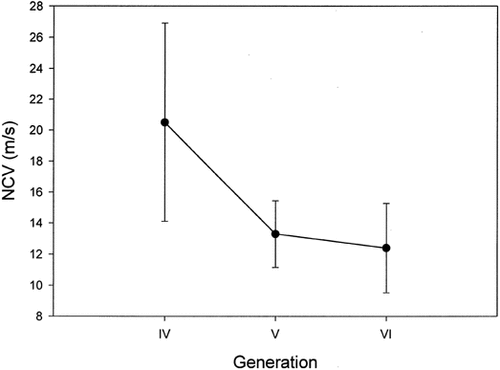
Graph representing the mean conduction velocities in different generations.
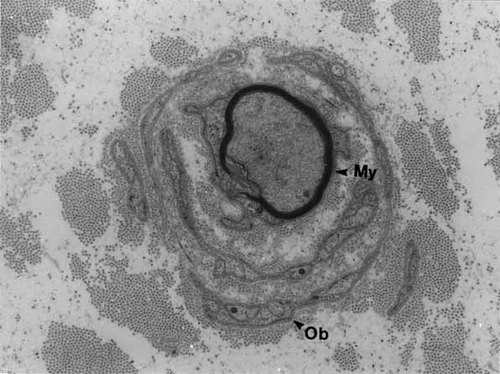
Electron microscopy of a sural nerve from the mother of the proband revealed “onion bulb” (Ob) whorls composed of multiple Schwann cell lamellae surrounding thinly myelinated axons (My).
Nerve conduction velocities were recorded from the nerves listed in Table I. Conduction velocities were not recorded on all nerves for all subjects. Therefore, mean nerve conduction velocities for each subject were used as raw data. Mean nerve conduction velocity was 20.5, 13.3, and 12.4 m/s, respectively, for generations IV, V, and VI. Again, the youngest generation was not included in the analysis for the above-stated reasons. Although the differences were not statistically significant, nerve conduction velocities in this population of patients showed deterioration with younger generations, an opposite trend to what one would expect for ascertainment bias.
| Nerve | n = 22 Mean conduction velocity (m/s) | Range m/s |
|---|---|---|
| Median motor | 14 | 9–20 |
| Ulnar motor | 13 | 9–16 |
| Median sensory | 18 | 10–33 |
| Ulnar sensory | 14 | 9–18 |
| Peroneal motor | 17 | 15–20 |
| Tibial motor | 11 | 9–14 |
The repeat expansion detection (RED) assay was employed to examine the molecular basis of clinical anticipation. A generational expansion of triplet repeat sequences evaluated did not correlate with clinical severity, nor were differences in repeat length noted between the maternal and paternal transmission lines. The RED assay, however, is a genomic screen of triplet repeat lengths and small disease-associated expansions can be masked by variable repeat lengths in other regions of the genome not associated with disease. The molecular marker D17S918 was recently mapped to the CMT1A duplication region on chromosome 17p and contains a polymorphic CAG repeat that has been hypothesized to be related to clinical anticipation observed in some families with CMT [Inoue et al., 2001]. Examination of the number of CAG repeats for individuals in this family failed to identify any expansion of the triplet repeat in affected family members [Inoue et al., 2001]. Further attempts to show a molecular association with disease manifestation by PCR analysis of alternative repeat elements identified within the genomic sequence of PMP22 were also unsuccessful.
DISCUSSION
Anticipation
Two previous studies have documented possible anticipation in CMT and deafness. Hamiel et al. [1993] described a family who demonstrated sensorineural deafness occurring before the development of overt signs of CMT. The age of onset of the disease in the family varied in affected members, with the presenting symptoms occurring earlier in each generation. A definitive anticipation pattern was not shown statistically in this family, however, as the number of affected members was too small. Molecularly, no abnormality of the chromosome segment at locus 17p11.2 was found in this family. Marrosu et al. [1997] reported a family that exhibited an extremely variable phenotype, ranging from a mild subclinical form in the older subjects to a severe and early disabling disease in the last-generation patients, suggesting an “anticipation-like” pattern. They found a point mutation in exon 5 of PMP22 causing dominant CMT1A disease. They suggest that the point mutation in the third domain of PMP22 protein determines CMT1 and the severity of the clinical phenotype can be modified by a second locus.
Roa et al. [1993] postulated Dejerine-Sottas (DSS) and CMT1A may more accurately represent a spectrum of related clinical findings due to allelic heterogeneity. The features of the proband in our family are more typical of DSS phenotype with hypotonia, absent reflexes, feeding problems, and respiratory distress. A mutation in the second allele was not identified to explain her severity. Mutation analysis of autopsy tissue in her deceased cousin indicated that he also had a dominant mutation in the PMP22 gene. With the clinical spectrum of molecularly related neurological disorders comes a significant variability in the clinical phenotype among individuals carrying identical mutations. Marques et al. [1999] also reported significant phenotypic variation resulting from a novel P0 point mutation in a pair of monozygotic twin brothers with CMT type 1B. One brother developed onset of his disease at 18 months and the other one at school age, with electrophysiological differences also noted of severity of axonal loss, suggesting other genetic or environmental factors to explain this difference.
Although only a few reports have documented anticipation in CMT1A within the same family resulting from a single point mutation in the PMP22 gene, precedence has been set with a 20-year longitudinal study among 13 affected members in four generations of the original family with CMT1B associated with a novel P0 mutation in whom the degree of severity and disability appeared to increase in each generation [Bird et al., 1997]. The majority of affected individuals in the youngest two generations developed disease by age 6 years. Age of onset was 18 years in the grandfather, with the latest onset of 30–40s in the great-grandmother. Our study reports a similar finding in a large family comprised of 34 affected individuals in whom the severity of disease in later generations appeared more dramatic, leading to demise in childhood in the proband and her cousin.
The vast majority of classical CMT1 is associated with duplication of PMP22; however, a few are associated with point mutations in PMP22. Mutation screening of the PMP22 gene [Nelis et al., 1996] indicated a 4.1% mutation rate. The clinical phenotype of persons carrying a PMP22 point mutation ranges from mild (HNPP or hereditary neuropathy and pressure nerve palsies) to very severe (Dejerine-Sottas syndrome, DSS). Nelis et al. [1999] and Kovach et al. [1999] summarized the genotype–phenotype correlation in CMT. The HNPP phenotype is associated with microdeletions and insertions, splice site mutations, and nonsense mutations, which result in expression of an aberrant, nonfunctioning protein [Bort et al., 1997]. Thirteen distinct heterozygous mutations (three in codon 72, which appears to be a mutation hotspot) are associated with DSS syndrome. At codon 79, two different amino acid substitutions lead to DSS or CMT1. It was concluded that although PMP22 point mutations are more likely to result in a severe clinical outcome than what is observed from gene duplication, there is no defined category of PMP22 point mutations that can distinguish between classical CMT and DSS. Two genetically distinct mutations in the transmembrane domain 2 of PMP22 have been previously reported in two isolated cases with hearing loss. One of these cases was a 32-year-old female who was severely affected with DSS with facial weakness, scoliosis, nystagmus, and nerve conduction velocity of 11 m/s. Her hearing loss was attributed to acquired causes [Ionasescu et al., 1996]. The Ser72 Leu mutation in her has been reported in three other cases without hearing loss. The other case [Tyson et al., 1997] was also a de novo case of DSS, a woman age 39 years; details of audiological studies are not available in this case report. Since the majority of CMT and DSS cases are not associated with deafness, molecularly evaluating those cases that display hearing loss is of interest, in order to define potential protein domains of PMP22 that interact and function in nonneural tissues such as the cochlea. We later recruited 25 individuals from 20 families with CMT and deafness through the Muscular Dystrophy Association and Charcot-Marie-Tooth Association. All were tested for the Ala67Pro mutation and found to be negative. The families were not large enough to perform a linkage analysis. Five individuals were found to have the common PMP22 duplication by fluorescent in situ hybridization. Four individuals with early onset deafness had sequencing performed of all coding exons of the PMP22 gene. Only one individual was found to have a polymorphism, a C to A transversion in the second intron (IVS2 + 7; ACGgtgaggc → ACGgtgagga). This alteration is just outside of the consensus sequence (in italics) of the 5′-splice site and therefore is not predicted to effect mRNA processing.
CMT and Deafness
Most prior audiologic studies of patients with CMT disease and deafness suggested that the hearing impairment may be entirely neural [Raglan et al., 1987; Gadoth et al., 1991; Hamiel et al., 1993], but in our family a concomitant cochlear component is present. However, in many of the previous reports of CMT and hearing loss the auditory studies described only “sensorineural hearing loss,” with no testing to clearly distinguish between cochlear and neural components.
Triantafyllou et al. [1989] reported that some CMT patients had prolonged ABR interpeak latencies, suggestive of retrocochlear disorder, while others showed delayed Wave I latency, suggestive of peripheral disorder. Perez et al. [1988] also reported ABR abnormalities with CMT disease but an incidence of only 28% patients with hearing loss. Although some of the ABR abnormalities reported in those studies are consistent with auditory neurologic abnormalities and elevated hearing thresholds, without OAE testing whether or not a concomitant cochlear component existed cannot be determined.
Fukada [1993] reported a single case of CMT type I with vocal cord palsy, hearing loss, diaphragmatic weakness, and cerebellopontine atrophy in a 42-year-old man from Japan. In this case the ABR was absent but because OAEs for cochlear function were not performed; it cannot be determined if the ABR was absent secondary to peripheral hearing loss, neurologic abnormality of the auditory pathway, or a combination thereof.
Boltshauser's family [1989] with motor-sensory neuropathy, vocal cord paralysis, and sensorineural hearing loss in three individuals in three generations of a large family had ABR test results that were consistent with normal neuro-otologic function and thus the authors concluded that the sensorineural hearing loss was cochlear rather than retrocochlear in origin. However, without OAEs to assess cochlear function, the interpretation is not fully conclusive. Young and Harper [1980] reported a larger kindred with similar neurologic abnormalities (individuals, however, had normal sensation and normal nerve conduction velocities) and vocal cord paralysis but with normal hearing. However, ABR testing for neurologic auditory abnormality was not performed nor were OAEs for early cochlear changes examined. Consequently, subclinical auditory abnormality could not be ruled out. Other studies have also reported normal or “near normal” ABR findings in CMT disease [Cassandro et al., 1986].
Additionally, a disparity between hearing threshold and the patient's self-report of hearing difficulty has been reported to indicate a neural component to the difficulties encountered by CMT patients [Musiek et al., 1982]. That disparity would be consistent with our finding in this family that word recognition is frequently poorer than would be predicted from auditory threshold alone.
Gadoth et al. [1991] reported 16 patients in five families who underwent trimodality evoked potential studies. History of hearing loss was present in three patients and sensorineural hearing loss was found in five of seven patients in whom audiometry was obtained. However, in these patients one of the ABR abnormalities was a prolongation of ABR Wave I latency, which is suggestive of a peripheral hearing loss component rather than neuro-otologic involvement. Because OAEs were not performed, the possibility that the ABR abnormalities may have been attributable, at least in part, to a peripheral problem cannot be addressed.
More detailed studies have suggested that CMT with deafness is neurologic in origin, as indicated by absent ABRs but present OAEs. Starr et al. [1996] reported on 10 individuals with hearing deficits and normal OAEs and absent ABRs. Eight of these individuals developed peripheral neuropathy, three were considered to have CMT type I, and one CMT type II.
In our family, however, both OAEs and ABRs were consistently abnormal, again suggesting auditory differences among families with CMT and deafness. Further detailed auditory studies will be needed in the various families with CMT and deafness to more clearly define the apparently heterogenous auditory phenotypes.
Although previous authors have noted clinical variability in CMT in different generations, this is the first report of a statistical analysis of anticipation of the onset of CMT, nerve conduction velocity, and deafness in a large central Illinois family with type 1 CMT associated with a unique point mutation in PMP22. A molecular mechanism could not be demonstrated to explain this phenomenon; however, as yet unidentified genetic and environmental factors may be responsible for this observation. Review of the literature indicated that deafness is not uncommon in CMT. We therefore recommend that medical providers consider testing for hearing loss in their patients with CMT.
Acknowledgements
We thank the family members and their physicians for participation in this study; Dr. James Lupski and Dr. Ken Inoue, Baylor College of Medicine, Houston, TX for analysis of intronic repeats in our family, Dr. Charles Eberle, Southern Illinois University School of Medicine, for obtaining the sural nerve; Dr. J.J. Martin, Dr. C. Ceuterick at the Laboratory of Neurogenetics, University of Antwerp, Belgium sural nerve interpretation; Nat Mody, Memorial Medical Center, Springfield, IL for sural nerve electron microscopy studies, Dr. Michael Koob (University of Minnesota) for methodology of RED assays; Ms. Lisa Johnson, Dr. Paneeya Sudabutra, Dr. Leonard Maroun, and secretarial staff at Southern Illinois University School of Medicine for their contributions.




