Prediction by FISH analysis of the occurrence of Wilms tumor in aniridia patients
Abstract
Aniridia is an autosomal dominant eye anomaly caused by haploinsufficiency of the PAX6 gene, of which abnormalities include base alterations, position effects and deletions. When deletion involves its adjacent genes, i.e., those in the PAX6-WT1 critical region (WTCR), patients are predisposed to Wilms tumor. We studied 18 patients with aniridia, five of whom had chromosome deletion involving 11p13, two a translocation t(10;11)(p13;p13) or a der(14;21)(q10;q10)mat, and 11 had a normal karyotype. Fluorescence in situ hybridization (FISH) using four P1-derived artificial chromosome (PAC) clones located at WTCR was carried out in the 18 patients to identify a deletion extent. Of the 18 patients, eight had a deletion of WTCR: four had microscopic deletion and four a deletion of WTCR. Deleted region in one patient with a microscopic deletion was distal to the critical region. Four of the eight patients with a deletion encompassing WTCR developed Wilms tumor, and the other four did not (two were too young to be evaluated for the tumor development). The data in the present study, together with four similar previous works, indicate that of a total of 102 aniridia patients, 29 had a deletion spanning WTCR. Wilms tumor developed in 13 (45%) of the 29 patients, whereas patients without deletion in this region did not develop the tumor. In other words, aniridia patients with WT1 deletion run a high risk of developing Wilms tumor, and those without the deletion do not. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Aniridia is a congenital eye malformation characterized by complete or partial absence of the iris. It is frequently associated with cataracts, macular hypoplasia, optic nerve hypoplasia and glaucoma, resulting in vision reduction and nystagmus [Olitsky and Nelson, 2000]. Aniridia is inherited in an autosomal dominant fashion with a population frequency of 1:60,000 to 1:100,000 [Shaw et al., 1960]. Aniridia and Wilms tumor are causally related: one-third of patients with aniridia have a potential of developing Wilms tumor [Fraumeni and Glass, 1968], and about 2% of those who develop Wilms tumor have aniridia [Shannon et al., 1982]. Some patients with 11p13 deletion present with aniridia, Wilms tumor, genitourinary abnormalities and mental retardation, i.e., WAGR syndrome [Narahara et al., 1984; Moore et al., 1986]. The gene, WT1, was identified as that responsible for Wilms tumor [Call et al., 1990; Gessler et al., 1990] and PAX6 (paired box gene 6) as that for aniridia [Ton et al., 1991], both located at distal 11p13. Aniridia is caused by haploinsufficiency of PAX6, resulting from sequence alterations, deletions or position effect. When gross deletions span the PAX6-WT1 region, the patient is predisposed to Wilms tumor. Thus, it is important to know a risk of developing Wilms tumor among patients with aniridia.
We report here the result of FISH analysis on the PAX6-WT1 region to understand the relationship between the extent of deletions and clinical features in 18 patients with aniridia.
PATIENTS AND METHODS
Patients studied included eight girls (Patients 1–2, 6–7, 10, 12–13, and 15) and 10 boys (Patients 3–5, 8–9, 11, 14, 16–18), aged 5 months to 17 11/12 years (mean, 5 11/12 years). All 18 patients were sporadic, and had aniridia and associated eye abnormalities, including visual reduction, nystagmus and cataracts. Their clinical manifestations are summarized in Table I. Four patients (Patients 1–4) had developed Wilms tumor. Patients 5 and 8, aged 18 and 11 months, respectively, were too young to be evaluated for the tumor development. Genitourinary anomalies were found in four of the 10 boys, including cryptorchidism, hypospadias, hypoplasia of the left kidney, and urethral stricture or Mullerian duct cyst; and in two girls, including renal cysts, streak gonads, and bicornuate uterus. Mild to moderate developmental delay was observed in eight patients. Of the 18 patients, five (Patients 2, 3, and 5–7) had chromosome deletion involving 11p13, one (Patient 1) a t(10;11)(p13;p13) and one (Patient 4) a der(14;21)(q10;q10)mat. The other 11 patients were karyotypically normal.
| Patient no. | Gender | Age (years) | Wilms tumor | Genitourinary anomalies | DR | Other abnormalities | Karyotypes | Copy number for marker | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAX6 | D11S2163 | PER | WT1 | ||||||||
| 1 | F | 3/12 | + | Streak gonads, bicornate uterus | + | − | 46,XX,t(10;11)(p13;p13) | 1 | 1 | 1 | 1 |
| 2 | F | 2 | + | ? | ? | ? | 46,XY,del(11)(p11.2;p13) | 1 | 1 | 1 | 1 |
| 3 | M | 2 | + | Hypoplasia of left kidney, urethral stricture | + | − | 46,XY,del(11)(p11.2;p14) | 1 | 1 | 1 | 1 |
| 4 | M | 4 1/2 | + | − | − | − | 45,XY,der(14;21)(q10;q10)mat | 1 | 1 | 1 | 1 |
| 5 | M | 1 6/12 | − | Cryptorchidism, hypospadias, renal cysts | + | Cardiomyopathy | 46,XY,del(11)(p11.2;p13) | 1 | 1 | 1 | 1 |
| 6 | F | 7 | − | Renal cysts | + | 46,XX,del(11)(p13p;14.3) | 1 | 1 | 1 | 1 | |
| 7 | F | 5 1/2 | − | − | − | Febrile convulsions, hyperextensible joints | 46,XX,del(11)(p13 p;14) | 2 | 2 | 2 | 2 |
| 8 | M | 11/12 | − | Cryptorchidism, hypospadias, renal cysts | + | − | 46,XY | 1 | 1 | 1 | 1 |
| 9 | M | 17 11/12 | − | Mullerian duct rest, cryptorchidism, hypospadias, urethral stricture | + | Chronic renal failure | 46,XY | 1 | 1 | 1 | 1 |
| 10 | F | 5/12 | − | − | + | Partial choroid defect | 46,XX | 2 | 2 | 2 | 2 |
| 11 | M | 1 1/12 | − | − | − | 46,XY | 2 | 2 | 2 | 2 | |
| 12 | F | 4 5/12 | − | − | − | 46,XX | 2 | 2 | 2 | 2 | |
| 13 | F | 4 7/12 | − | − | − | 46,XX | 2 | 2 | 2 | 2 | |
| 14 | M | 6 | − | − | − | 46,XY | 2 | 2 | 2 | 2 | |
| 15 | F | 6 1/12 | − | − | − | 46,XX | 2 | 2 | 2 | 2 | |
| 16 | M | 8 7/12 | − | − | − | 46,XY | 2 | 2 | 2 | 2 | |
| 17 | M | 9 | − | − | + | Epilepsy, inguinal hernia, hydrocephalus | 46,XY | 2 | 2 | 2 | 2 |
| 18 | M | 11 1/4 | − | − | − | 46,XY | 2 | 2 | 2 | 2 | |
- DR, development retardation; M, male; F, female; +, presence; −, absence; ?, unknown.
FISH Analysis
Chromosome preparations were made from peripheral blood T lymphocytes, Epstein-Barr virus-transformed lymphoblastoid cells or cultured skin fibroblasts of patients. FISH was performed using four P1-derived artificial chromosome (PAC) clones (1083G3 for PAX6; 65P5 for D11S2163; 685F3 for PER; and 104M13 for WT1) as probes [Niederfuhr et al., 1998]. The localization, order, and the size of these clones are shown in Figure 1. The probes were biotin-labeled and hybridized to metaphase chromosomes, as described elsewhere [Ohashi et al., 1994]. Then, the chromosomes were counterstained with PI, and photomicroscopy was carried out under an epifluorescence microscope. Probe D11Z1 (Oncor Inc., Gaithersburg, MD) was used as a control to identify chromosome 11.
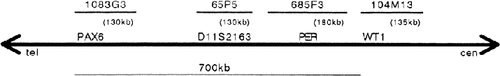
Physical map of an 11p13.3 region to which four loci/clones (probes) are located.
RESULTS AND DISCUSSION
FISH analysis showed that four of the five patients with 11p13 deletions lacked all four PAX6-WT1 loci, i.e., the Wilms tumor critical region (WTCR), whereas the other patient (Patient 7) retained WTCR, an indication that her microscopic deletion was distal to the critical region. Two (Patients 8 and 9) of the 11 patients with a normal karyotype had an interstitial deletion encompassing WTCR. The two patients (Patients 1 and 4) with a translocation had a deletion involving WTCR (Table I). Altogether, eight of the 18 patients had a deletion of WTCR (Fig. 2).
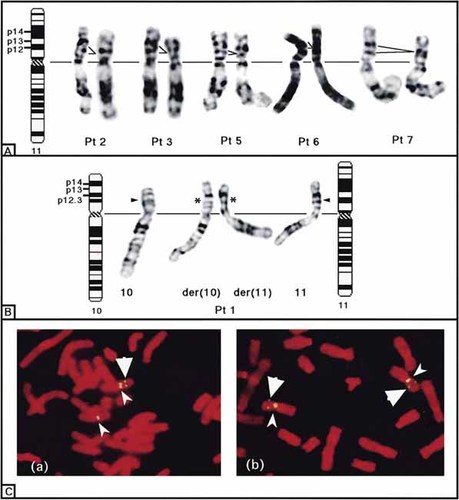
Deletions at 11p13 detected by G-band karyotyping of chromosome 11 from five patients (A) and a patient with t(10;11)(p13;p13) (B), and by FISH (C). Large and small arrowheads indicate WT1 and centromere, respectively. a, heterozygous WT1 deletion; b, no deletion.
Comparison of clinical manifestations with the results of FISH analysis revealed that of the eight patients with deletion of WTCR, four (Patients 1–4) developed Wilms tumor (Table I). Patients 5 and 8, aged 18 and 11 months, respectively, were too young to be evaluated for the tumor development. When combined our 18 patients with 84 patients in four previous studies [Mannens et al., 1991; Fantes et al., 1992; Drechsler et al., 1994; Crolla et al., 1997], a total of 102 aniridia patients have been analyzed for WT deletion. In view of deletion extents, they are divided into the following three groups: Group 1 consists of 69 patients without deletion of WTCR, Group 2 includes four patients with PAX6 deletion but not involving WT1, and Group 3 is composed of 29 patients with deletion involving both PAX6 and WT1. No patients in Groups 1 or 2 developed Wilms tumor, whereas 13 (45%) patients in Group 3 developed the tumor. Thus, aniridia patients with WT1 deletion run a high risk of developing Wilms tumor, and those without the deletion do not. These findings support that haploinsufficiency of PAX6 causes aniridia, and loss of one WT1 alleles can comprise the first hit in the sequence of events to cause Wilms tumor. Renal ultrasonography is recommended every 3 months to detect Wilms tumor in aniridia patients. According to the data from 61 patients with WAGR syndrome by Beckwith [1998], cumulative percentage of Wilms tumor diagnosed per year was 64% by 2 years, 90% by 4 years, 98% by 7 years, and 100% by 8 years. Thus, a reasonable stopping point for routine ultrasonographical surveillance in children with WT1 deletion would be the end of age 7 years.
Genitourinary anomalies were observed in six of the eight patients with WT1 deletion. The anomalies in two such girls included streak gonad, bicornate uterus (Patient 1) and a renal cyst (Patient 9), and those in four boys were hypospadias and cryptorchidism (Patients 5–6 and 7), urethral structure (Patients 3 and 7), renal cysts (Patient 5) and unilateral renal atrophy (Patient 3). Patient 7 developed secondary chronic renal failure, probably as a result of external urethral orifice stenosis and the residual cyst of the Mullerian duct. Genitourinary abnormalities were not observed in any patients without WT1 deletion. These findings indicate that WT1 is crucial for genitourinary development in embryogenesis [Kreidberg et al., 1993].
Mild to moderate developmental delay was present in eight patients, six of which had either or both of microscopic and molecular deletion involving WTCR. Three of the eight patients had 11p13 deletion, three lacked WTCR, and two (Patients 10 and 17) retained the critical region. These observations suggest that patients with deletion of WTCR have a high risk of having developmental retardation. Developmental delay in Patient 17 was most likely secondary to perinatal problems, such as severe gestosis and neonatal hypoglycemia. There was no apparent reason for the developmental delay in Patient 10 (Table I).
Acknowledgements
We thank Drs. S. Saitou, S. Sasaki, J. Kimura, K. Imaizumi, M. Matsuo, Y. Nozaki, Y. Yamada, and Y. Fukushima for providing patient information.




