Intra-tumoral and peripheral blood TIGIT and PD-1 as immune biomarkers in nodular lymphocyte predominant Hodgkin lymphoma
Jay Gunawardana and Soi C. Law are contributed equally.
The findings were presented partially by J.G. at the 2nd American Association for Cancer Research (AACR) Malignant Lymphoma meeting in Boston, MA (2022) and at the 63rd annual meeting of the American Society of Hematology (ASH) in Atlanta, GA (2021) which was recognized by an ASH Abstract Achievement Award.
Abstract
In classical Hodgkin lymphoma (cHL), responsiveness to immune-checkpoint blockade (ICB) is associated with specific tumor microenvironment (TME) and peripheral blood features. The role of ICB in nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is not established. To gain insights into its potential in NLPHL, we compared TME and peripheral blood signatures between HLs using an integrative multiomic analysis. A discovery/validation approach in 121 NLPHL and 114 cHL patients highlighted >2-fold enrichment in programmed cell death-1 (PD-1) and T-cell Ig and ITIM domain (TIGIT) gene expression for NLPHL versus cHL. Multiplex imaging showed marked increase in intra-tumoral protein expression of PD-1+ (and/or TIGIT+) CD4+ T-cells and PD-1+CD8+ T-cells in NLPHL compared to cHL. This included T-cells that rosetted with lymphocyte predominant (LP) and Hodgkin Reed–Sternberg (HRS) cells. In NLPHL, intra-tumoral PD-1+CD4+ T-cells frequently expressed TCF-1, a marker of heightened T-cell response to ICB. The peripheral blood signatures between HLs were also distinct, with higher levels of PD-1+TIGIT+ in TH1, TH2, and regulatory CD4+ T-cells in NLPHL versus cHL. Circulating PD-1+CD4+ had high levels of TCF-1. Notably, in both lymphomas, highly expanded populations of clonal TIGIT+PD-1+CD4+ and TIGIT+PD-1+CD8+ T-cells in the blood were also present in the TME, indicating that immune-checkpoint expressing T-cells circulated between intra-tumoral and blood compartments. In in vitro assays, ICB was capable of reducing rosette formation around LP and HRS cells, suggesting that disruption of rosetting may be a mechanism of action of ICB in HL. Overall, results indicate that further evaluation of ICB is warranted in NLPHL.
1 INTRODUCTION
In the 5th edition of the World Health Organization Classification of Haematolymphoid Tumours, Hodgkin lymphoma (HL) comprises two distinct disease entities: classical Hodgkin lymphoma (cHL) and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL).1 The histological hallmark of both are a malignant B-cell infiltrate that makes up only approximately 2% of the diseased node. However, there are also histological differences. cHL consists of four subtypes, the malignant Hodgkin Reed–Sternberg (HRS) cells are typically interfollicular, lack a functional B-cell receptor (BCR), and are CD30+.1 In contrast, NLPHL has six “Fan” subtypes A-F, the malignant “lymphocyte predominant” (LP) cells in NLPHL are typically located within follicles, have an intact BCR, and are CD20+.2 Typical (A+B) NLPHL have a superior prognosis to atypical (C-F) subtypes.3 The biological similarities of NLPHL with indolent B-cell lymphomas are reflected in its alternative naming of “NLP B-cell lymphoma” by the International Consensus Classification of Mature Lymphoid Neoplasms.4 Although historically NLPHL has been frequently treated with the same chemotherapies used in cHL, therapeutic strategies are beginning to diverge between the two HL entities.5-8 cHL has a bimodal age distribution, is aggressive, and 75% are advanced stage,9 whereas NLPHL (<5% of all HL) has a median age of approximately 40 years,2, 10 is indolent, typically early stage, and late transformation to aggressive non-Hodgkin lymphomas is well-described.11-13 Both disorders appear to have a relationship with pathogens in a subset of cases, but the nature of these pathogens is different.14, 15
There are few NLPHL genomic sequencing analyses, with studies revealing frequent structural alterations in BCL6 and recurrent mutations in DUSP2, SGK1, and JUNB.5 However, in contrast to cHL,16-18 copy number alterations involving programmed cell death-1 ligand PD-L1, β2M, and CIITA do not appear to be prominent.19 Whereas human leukocyte antigen-II (HLA-II) is frequently reduced in cHL, it is retained in NLPHL.20, 21
The tumor microenvironment (TME) of cHL serves as a paradigm for the bidirectional interactions between the TME and HRS cells.9, 22, 23 Understanding this interplay has underpinned the proven clinical efficacy of immune-checkpoint blockade (ICB) to boost anti-tumoral immunity in cHL.24-29 cHL's TME is relatively well-characterized and associates with outcome after conventional therapy.30-32 Furthermore, the T-cell immune signature of cHL peripheral blood is also associated with responsiveness to ICB.33 With NLPHL, a large study showed PD-1+CD4+ T-cells frequently rosette around LP cells.34 Another study of 15 NLPHL cases showed reduced numbers of CD4+ regulatory T-cells (Tregs) compared to four cases of lymphocyte rich cHL.35 Otherwise, little is known regarding the TME or peripheral blood T-cells in NLPHL, particularly detailed multiomics or functional data about potentially clinically actionable TME targets. This has hampered the evaluation of immunotherapeutic drugs that potentiate host anti-tumoral immunity in NLPHL.
Given that the TME in lymphomas is influenced by the immune responses elicited by malignant cells, the TME in NLPHL is likely to be distinct from cHL. There remains a need to compare the TME-driven contributions to immune-evasion between the entities. Such a study might provide a preclinical rationale to establish if ICB may also treat NLPHL. Here, we compared the immune landscape of NLPHL and cHL tumors with a focus on clinically pertinent immune-checkpoints.
2 METHODS
2.1 Patient samples
Diagnostic formalin-fixed paraffin embedded (FFPE) samples from 121 NLPHL and 114 cHL patients were examined. Only de novo cases were included. HIV-positive and organ-transplant patients were excluded. Patient selection was based on sample availability. NLPHL histological subtyping was performed in line with published criteria,36 by accredited histopathologists (S.B. (Birch), C.S. (Snell), P.F.). T-cell/histiocyte rich B-cell lymphomas were excluded. Patient age, sex, and stage of the combined cohorts (Table S1) are in line with published data, including a preponderance of male sex for NLPHL.10 Consort diagrams with detailed sample breakdown of the discovery and validation cohorts are available (Figure S1A–C). Ten non-lymphomatous/nonreactive nodes (NLN) (median 53 years, range 41–69), and 11 reactive/non-lymphomatous nodes (RLN) (median 48 years, range 14–73), sourced from surgically excised lymph nodes all without histological evidence of any malignancies, were included as controls. Pre-therapy peripheral blood mononuclear cells (PBMCs) were available from 14 NLPHL, 12 cHL, and 10 healthy participants. Pre-therapy plasma from 13 NLPHL, 13 cHL, and 13 healthy participants (a different control group to the NLN/RLN participants) were collected before therapy initiation (pre). Paired plasma collected at 6 months post-therapy (post) in six NLPHL and 11 cHL cases were available. This study was approved by the relevant institutional regulatory boards and was performed in concordance with the Declaration of Helsinki.
2.2 Multiomic assays
RNA was digitally quantified using the nanoString® PanCancer Immune panel (730 immune-related genes plus 40 housekeeping genes), as previously published.37 Normalized gene counts were deconvoluted with CIBERSORTx Fractions model using the LM22 signature matrix, with 1000 permutations and B-mode batch correction.38-40 Ten NLPHL and eight cHL FFPE tumors and paired pre-therapy PBMC samples were chosen for T-cell receptor repertoire (TCR) sequencing. Tissue samples were chosen based on availability of sufficient DNA and paired blood. TCRβ CDR3 regions were amplified from between 400 ng and 1 μg of input DNA using the immunoSEQ human TCRB kit (V4.0; Adaptive Biotechnologies), as published.41, 42 Flow cytometry for T-cell subsets was performed on all available PBMCs (the gating strategy is shown in Figure S2 and antibody panel in Table S2).
Multispectral microscopy (Opal, Akoya Biosciences) and multiplexed tissue-microarray (Phenocycler, Akoya Biosciences) were performed as outlined in the Supplement, with Table S3 and Figure S6A–D providing details of the antibody panels used.
2.3 Functional assays of PD-1 and TIGIT blockade
The rosetting assay was adapted from a published protocol that was originally designed to investigate rosetting in cHL.43 The number of rosettes (defined as a cluster containing one tumor cell and ≥3 adherent lymphocytes) were quantified in a tumor cell-line/PBMC co-culture model treated with anti-PD-1 antibody alone, anti-TIGIT antibody alone, dual anti-PD-1/TIGIT antibodies, or relevant isotype controls. For cHL, PBMC HLA-II matched to the KM-H2 tumor cell-line were used, whereas unmatched were used for the NLPHL DEV (HLA-I and HLA-II absent) cell-line. See Data S1 for experimental details.
2.4 Statistical analysis
Comparisons between groups of data were tested for statistical significance using a Welch t-test, Mann–Whitney rank sum test, or Dunn's multiple comparisons test, as appropriate (GraphPad Prism 9). All tests were two-sided at the threshold of p = .05. Multiple testing using the Benjamini–Hochberg false discovery (FDR) method was performed where indicated.
2.5 Data sharing
NanoString data is in a data supplement available with the online version of this article.
3 RESULTS
3.1 TIGIT and PD-1 are more elevated in NLPHL relative to cHL
Therapeutic modulation of the TME is beneficial in cHL but remains untested in NLPHL. To gain insights into its potential in NLPHL, we initially quantified the immune landscape in 110 HL tumors (discovery cohort, center dependent) by digital RNA hybridization. Fifty-six NLPHL and 54 cHL were examined. Sixteen randomly selected samples were rerun to control for batch effects and gene counts were within 10% of runs. In order to confirm the discovery cohort findings, digital RNA hybridization using the same immune gene panel was applied to a validation cohort (new centers) of 125 HL diagnostic tissues (65 NLPHL vs. 60 cHL). Six samples from the discovery cohort were rerun along with the validation cohort and showed strong concordance between runs (r2 = .956, p < .001).
After adjusting for false discovery, Figure 1A,B illustrates the significant differentially expressed genes (DEGs) between the two diseases in discovery and validation cohorts. Figure 1C shows the volcano plot for the combined cohorts (121 NLPHL vs. 114 cHL tumors). Among the most consistently striking upregulated DEGs in NLPHL relative to cHL found in discovery, validation and combined cohorts included the T-cell associated immune-checkpoint genes ITIM domain TIGIT and PDCD1 (PD-1), which showed 2.3-fold and 2.4-fold enrichment, respectively. Among the other most differentially over-expressed genes in NLPHL versus cHL (Figure S3) were the T-cell related genes CD247: CD3 zeta chain; GZMK: granzyme K; and CD28: a co-stimulatory molecule. As expected, across cohorts there was consistent enrichment for CD30 and CCL17 (TARC) in cHL.
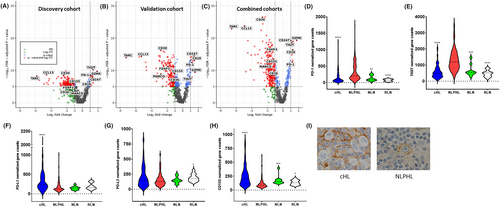
Based on these findings, we focused on immune-checkpoint gene expression, comparing levels in the combined cohorts of NLPHL and cHL with NLN and with RLN. The comparative values for PD-1 and TIGIT were approximately 2–4.4-fold-higher for NLPHL versus NLN and RLN (Figure 1D,E). Immune-checkpoints CTLA4, BTLA, and LAG3 showed more modest differential expression, whereas there was no difference for TIM-3 (HAVCR2) or OX40 (TNFRSF40) (Figure S4A). Gene expression for the intracellular antigen presenting molecules CTSS, CD58, and NLRC5 were lower for cHL compared to NLPHL (Figure S4B).
To better put into context the impact of the relevant immune-checkpoints on their relevant signaling axis, we next tested for immune-checkpoint ligand gene expression (PD-L1/PD-L2 for PD-1; CD155 for TIGIT, Figure 1F–H). Given the known mechanisms of ligand amplification operative within HRS cells,44 as expected PD-L1 were lower in NLPHL than cHL (although PD-L2 values were similar). CD155 was also lower in NLPHL versus cHL. Immunohistochemistry (IHC) analysis showed that CD155 is present on the cell surface of LP and HRS cells (Figure 1I).
However, there were other genes that were consistently under-expressed in NLPHL relative to cHL. For example, in line with the lower levels of CCL17 in NLPHL (a chemokine that is known to induce migration of Tregs), there were significantly lower counts of the Treg marker FOXP3 in NLPHL than in cHL (Figure S3). Macrophage-associated scavenger receptor genes CD36, MARCO; and the macrophage-attractant chemokine gene CCL13 were also markedly lower in NLPHL. The Treg and macrophage findings are consistent with a recent multispectral microscopy analysis comparing NLPHL to four cases of lymphocyte rich cHL.35 Sub-analysis of DEGs, performed on the combined NLPHL and cHL cohorts, for early-stage versus advanced stage, histological subtype (Fan Type A vs. other, Type C vs. other, Type A+B [typical] vs. C-F (atypical)) and cHL (nodular sclerosing vs. other) histological subtypes, EBV status (for cHL only), and age (stratified by median age) in each disease showed no significant differences in gene counts (data not shown). The only exception was that HSD11B1 and CXCL11 were significantly downregulated in Typical versus Atypical. The biological relevance of this observation is unclear.
CIBERSORTx was used to deconvolute the normalized RNA counts and graphs were plotted for the major cell types (Figure S5). This showed elevation of NK-cells, granulocytes, dendritic cells, macrophages, monocytes, CD8+ T-cells, Tregs, and resting memory T-cells in cHL compared to NLPHL. Total CD4+ T-cells, naïve CD4+ T-cells, T-Follicular Helper (TFH) cell, and activated memory T-cells were higher in NLPHL.
Together the data indicate that the immune transcriptome is strikingly different between the two HL entities. With cHL there is enrichment in macrophage and Treg genes, whereas T-cell immune-checkpoints and associated genes are increased in NLPHL. Findings are strongly indicative that distinctly different immune-evasion strategies are operative between the two HL entities.
3.2 NLPHL is enriched for PD-1+ (and/or TIGIT+) CD4+ and PD-1+CD8+ T-cells compared to cHL
Although gene expression provides important quantitative data on the intra-tumoral immune landscape, it is unable to determine which genes are expressed within functionally distinct individual T-cell subsets. To gain insights into which T-cells expressed immune-checkpoints at a protein level, several overlapping approaches were adopted. Firstly, multispectral microscopy was used to test PD-1 and TIGIT expression on T-cells in 12 cHL and 19 NLPHL FFPE tissues (Figure 2A).
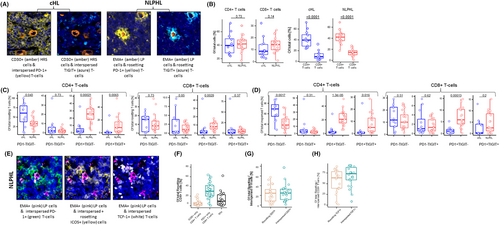
As expected, this confirmed that the majority of intra-tumoral T-cells for both HL entities were CD4+ (p < .0001 for each, Figure 2B). Between cHL and NLPHL there were no differences in the proportion of T-cells in direct contact (rosetting) with the malignant HL cells, with approximately 5–10-fold more CD4+ than CD8+ T-cells directly contacting either HRS or LP cells. However, there was differential expression of immune-checkpoints in T-cells that rosetted between the HL types. There was a marked and significant increase in the intra-tumoral PD-1+TIGIT-ve (5–10-fold), and PD-1+TIGIT+ (~4-fold) CD4+ and PD-1+TIGIT- (~3-fold) CD8+ T-cells in NLPHL compared to cHL (Figure 2C). Similar findings were observed in T-cells that were “interspersed” (i.e., not in contact) with LP and HRS cells (Figure 2D).
3.3 T-cells are enriched in the ICB response marker TCF-1 by multiplexed tissue-microarray
Whereas previous tissue studies at the single-cell level involving high throughput protein markers have been performed in cHL,25 data in NLPHL using these technologies are relatively lacking. To further interrogate the prominent levels of PD-1+ T-cells in NLPHL, the Phenocycler multiplexed tissue-microarray imaging procedure was used in 22 NLPHL tissues (Figure 2E).45
This showed that among CD4+ T-cells rosetting with LP cells, approximately 30% expressed PD-1, and approximately 10% co-expressed both PD-1 and ICOS, consistent with a classical TFH cell phenotype (Figure 2F). A similar proportion of interspersed CD4+ T-cells had a TFH phenotype. For both rosetting and interspersed T-cells with a TFH phenotype, the proportion that expressed CD57 was approximately 25% (Figure 2G). The majority (>80%) of both rosetting and interspersed TFH cells expressed TCF-1, a known marker of heightened T-cell ICB responsiveness.46 Approximately 67.5% of total CD4+ T cells were TCF-1+, and the proportion of PD-1+ rosetting and interspersed CD8+ T-cells that expressed TCF-1 was approximately 60%–70% (Table S4 and Figure 2H). There was no difference in memory T-cell subsets between rosetting and interspersed CD4+ or CD8+ T-cells (Figure S6E).
In distinction to previous findings in a series by Panayi et al. of 15 NLPHL cases imaged by multispectral microscopy,35 we did not observe progressive expansion of PD-1+ and GZMB+ CD8+ T-cells moving from A to E Fan subtypes (Figure S7). Nor were there differences in interferon-γ or TCF-1 expression in CD8+ T-cells by histology (not tested by Panayi).35 We also did not confirm their findings that PD-L1+ CD163+ macrophages were different between histological subtype. However, both Panayi and our study contain relatively small numbers of cases within each Fan histological subtype, and larger series are required before definitive conclusions can be made. In line with our nanoString findings and previously published flow cytometry,19 PD-L1 was rarely expressed by LP cells.
3.4 Expanded populations of clonal TIGIT+PD-1+CD4+ and TIGIT+PD-1+CD8+ T-cells circulate between the blood and TME
To examine circulating T-cell immunity, multi-parameter flow cytometry and dimensionality reduction (Figure S8A) were used to identify the principal blood T-cell subsets (CD8, TH1, TH2, TH17, or Treg). Distributions of PD-1 and TIGIT in CD4+ and CD8+ T-cells in pre-therapy NLPHL, cHL, and healthy participant blood were compared.
For CD8+ T-cells a higher proportion were dual PD-1+TIGIT+ in cHL versus healthy controls, whereas no difference was observed in NLPHL. However, for CD4+ T-cells a higher proportion of Treg, TH1, and TH2 subsets were PD-1+TIGIT+ in NLPHL versus cHL (Figure 3), but not for TH17 (Figure S9). TCF-1 was frequently expressed in TH1, TH2, TH17 T-cells, but not Tregs or CD8+ T-cells (Figure S10). As expected, within CD4+ T-cells, TCF-1 was inversely correlated with the exhaustion marker EOMES (Spearman r = −.55, p = .0004).
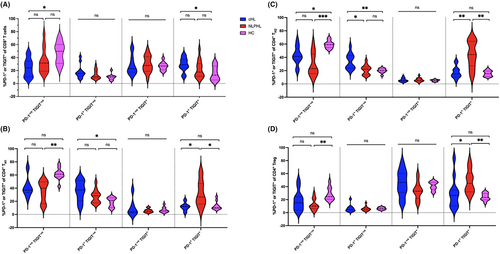
In view of recent data that checkpoint-ligands can be expressed on T-cells as well as monocytes,47 CD155/PD-L1/PD-L2 were also quantified (Figure S8B). However, this showed minimal T-cell and monocyte ligand expression with no differences between healthy control and HL entities, indicating that the circulation did not reflect the reduced PD-L1 and CD155 gene expression observed within NLPHL diseased nodes.
To establish whether there were populations of peripheral blood T-cells that circulated within the lymphatics and present within the HL TME, we used a recently developed approach.42 Here, TCR repertoire analysis was applied to paired intra-tumoral/blood samples, to identify T-cell clones that share the same TCR β-chain within both tumor and blood. Since β-chain sequencing alone cannot distinguish whether clones are CD4+ or CD8+ T-cells, the CD4/CD8 status of intra-tumoral T-cell clones was obtained by identifying shared T-cell clones within fluorescence-activated cell sorted (FACS) blood CD4+ and CD8+ T-cells, stratified by PD-1 and TIGIT expression (Figure 4A). In line with the multi-parameter flow cytometry data, there were a higher proportion of PD-1+TIGIT+CD4+ T-cells for NLPHL than cHL.
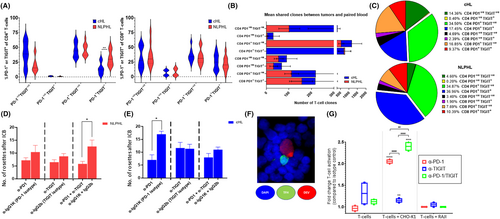
The mean proportion of intra-tumoral T-cell clones (i.e., present within the TME) that were shared with the blood were approximately 10% for both cHL and NLPHL. For cHL, shared intra-tumoral T-cell clones were at similar levels for CD4+ to CD8+; however, they were approximately 4x more CD4+ than CD8+ T-cells for NLPHL. For both HL entities, the majority of intra-tumoral CD4+ and CD8+ T-cell clones were predominantly PD-1+TIGIT-ve or PD-1+TIGIT+, with PD-1-veTIGIT-ve being next most frequent (Figure 4B). Next, we restricted our analysis to the most highly expanded shared T-cell clones (Figure 4C), as these clones are likely to have the greatest impact on HL immunobiology. For this, we tested the SharedCumFreq-100 (i.e., the most abundant 100 clonotypes of the shared T-cell clones between tumor and blood). For both HL entities, this again showed that the most abundant T-cell clones predominantly resided within PD-1+TIGIT-ve and PD-1+TIGIT+ subsets. Descriptive data is provided in Table S5.
Blood plasma was also tested. Soluble PD-1 but not TIGIT declined between pre- and post-therapy with both HLs (Figure S11).
3.5 Rosetting is impaired and T-cell activity enhanced by PD-1 and/or TIGIT immune-checkpoint blockade
A unique feature of HL is the presence of CD4+ T cells that surround and promote survival of tumor cells. To test if this interaction can be disrupted, we used an established in vitro rosetting model.43 Healthy participant PBMCs were co-cultured with the cHL-derived cell-line KM-H2 or the NLPHL-derived cell-line DEV (Figure 4D,E). For DEV, dual-blockade (but not single blockade for either PD-1 or TIGIT) led to a significant decrease in the number of rosettes compared to the relevant isotype control (mean 5.2 vs. 12.6; p = .038). For KM-H2, PD-1 blockade resulted in fewer rosettes (mean 7 vs. 17; p = .02). Results provide proof-of-concept that ICB is capable of reducing rosette formation around LP and HRS cells. CD57 staining demonstrated that a proportion of rosetting cells were TFH cells (Figure 4F).
As the rosetting assay included a mixed population of cells, to conclusively demonstrate that CD4+ T-cells are activated by antibody blockade of TIGIT and PD-1 ligands, we used a co-culture assay using target and effector cell-lines (Figure 4G). The PD-L1+/CD155+ CHO-K1 was the target and PD-L1-/CD155 Burkitt's lymphoma-derived Raji the negative control cell-line. Genetically engineered Jurkat CD4+PD-1+TIGIT+ reporter cells were used as the effector T-cell line. Results conclusively indicate that PD-L1/CD155 blockade induced T-cell activation measured by bioluminescence after treatment with either anti-PD-1 alone or anti-TIGIT alone and that dual anti-PD-1/TIGIT neutralizing antibodies further enhanced T-cell activation. There was no increase in bioluminescence with Raji or with Jurkat's alone.
4 DISCUSSION
We compared NLPHL and cHL using a transcriptomic discovery/validation approach followed by an integrated multiplex T-cell phenotypic, TCR, tissue, and blood analysis. Results indicate that immune-evasion mechanisms within the TME of NLPHL are distinct to those active in cHL, including marked increases in intra-tumoral protein expression of PD-1 (and/or TIGIT). Intra-tumoral and circulating PD-1+CD4+ T-cells (excluding Tregs) frequently expressed the transcription factor TCF-1, which has been shown to mediate proliferation of anti-tumoral T-cells with stem-like properties in response to ICB.46 Highly expanded T-cell clones in the TME expressing PD-1 and/or TIGIT were shared with the blood, demonstrating circulation of these T-cells between blood and lymphoid compartments. ICB was capable of disrupting rosette formation. Put together, the TME and blood findings indicate that NLPHL has multiple features that support further investigation of ICB in this disease.
A previous study using single-staining by IHC for TIGIT and PD-1 in cHL showed high expression of these immune-checkpoints in peri-tumoral lymphocytes.48 No comparison with normal nodes was provided. By contrast, a recent study of fresh intra-tumoral T-cells in cHL showed by flow cytometry that the expression of PD-1+ and TIGIT+ T-cells was similar to that observed in RLN.49 In concordance with the latter study, we observed by gene expression that for cHL, TIGIT and PD-1 levels were similar to that observed in NLN and RLN. The high resolution, dynamic range, and specificity of flow cytometry and digital gene expression may be one explanation for the discrepancy with the IHC study.
However, neither gene expression nor flow cytometry is capable of providing information on the spatial composition of T-cells within the diseased node. Previous studies involving spatial localization in cHL, other lymphomas, and solid cancers26, 50, 51 suggest PD-1+ T-cell topography is a predictor of ICB response. In the current study, there was marked increase in the proportion of intra-tumoral PD-1+CD4+ T-cells (including PD-1+/TIGIT+ cells) and PD-1+CD8+ T-cells in NLPHL compared to cHL. The increased immune-checkpoint expression included T-cells rosetting with LP cells.
Importantly, our study compared NLPHL to cHL (known to be responsive to ICB) and included analysis of both the TME and blood. Circulating immunity is required for effective immunotherapy in solid tumors,52 and in cHL the immune signature of peripheral blood T-cell clones is associated with responsiveness to PD-1 blockade.33 In pre-therapy blood, we observed a pronounced expansion of PD-1+TIGIT+ Treg, TH1, and TH2 subsets of patients with cHL compared to healthy participants. The expansion of these circulating PD-1+TIGIT+ T-cell subsets was more marked in NLPHL than cHL, suggesting that the blood signature in NLPHL has more pronounced features of ICB responsiveness than cHL.
Recent interrogation of the T-cell receptor on cHL intra-tumoral T-cells shows clonal expansions in Tregs and CD8+ T-cell subsets.49 Data linking circulating T-cell clones with paired intra-tumoral T-cell clones is lacking. In the present study, for both HL entities there were CD4+ and CD8+ T-cell clones shared between the blood and tumor that express PD-1 and/or TIGIT. For NLPHL, this was particularly marked for CD4+ T-cell clones. Many blood T-cell clones were highly expanded within the TME, that is, there are large T-cell clones expressing immune-checkpoints that circulate between blood and lymphoid compartments. Responsiveness to ICB may be directly through T-cells residing within the TME, as well as indirectly via shared T-cells that circulate from the periphery to the tumor.
Despite the proven efficacy of ICB, the mechanisms by which PD-1 blockade induce cHL regression remain incompletely understood. Given that TME is enriched in CD4+ T-cells and both HLA-II and a diverse CD4+ (but not CD8+) T-cell repertoire are predictive of higher response rates to ICB, it is likely that the anti-cHL immune response is predominantly mediated by CD4+ T-cells.33, 53 However, rapid response to ICB is not associated with activation of CD4+ effector T-cells,54 indicating that other T-cell mediated mechanisms are involved. Notably, longitudinal profiling of patients with cHL observed an increase in TIGIT and decline in PD-1 expression on T-cells upon successful treatment with the anti-PD-1 antibody nivolumab.55 This is broadly consistent with the sustained elevation of soluble TIGIT and drop in soluble PD-1 observed in patients with cHL treated with chemotherapy that we observed.
Histologically, rosetting T-cells are particularly prominent in NLPHL, but are also an established feature of cHL. However, definitive evidence of their biological role remains elusive. In keeping with the modest level of intra-tumoral PD-1+ and/or TIGIT+ T-cells in cHL, rosetting T-cells contained low levels of these immune-checkpoints. By contrast, substantially higher levels of PD-1+ and PD-1+/TIGIT+ rosetting T-cells in NLPHL were observed. The strong expression of PD-1 (and less frequently ICOS) indicate they likely originate from TFH cells.56-59 By multiplexed tissue-microarray we confirmed that approximately 10% of rosetting CD4+ T-cells had a PD-1+ICOS+ TFH phenotype and that T-cells (typically CD4+) without these phenotypic markers were also present. We demonstrate that ICB prevents CD4+ T-cell activation and that this was enhanced by dual-blockade. This, along with the high frequency of TCF-1 expressing T-cells rosetting with LP cells, suggests that ICB disruption of rosette formation might be a mechanism of action for NLPHL.46 Given their proximity to LP and HRS cells, it is possible that disrupting rosetting by T-cells reduces tumor cell support and/or physical shielding of HL cells from hostile immune cells.35 Sequential biopsies and/or rosetting assays in patients treated with ICB would assist in characterizing this further, to more clearly justify any future therapeutic approaches.
It is known in NLPHL that immune synapses form between HLA-II of LP cells and TCRs of rosetting TFH cells (consistent with provision of a growth-promoting signal).21 HLA gene expression was broadly similar between HL subtypes, whereas intracellular antigen presentation genes (involved in processing of peptides to the cell surface in the context of HLA molecules) was reduced in cHL compared to NLPHL. Further studies are required to fully characterize this observation. By immunochemistry, 10% of NLPHL cases have aberrant HLA-I and/or HLA-II expression.60 Therefore, were HLA-TCR interactions critical, ICB might be expected to work in the majority of NLPHL cases. However, this is almost certainly to be oversimplistic, because the same study found that 88% of cHL have aberrant HLA-I or HLA-II expression, yet this is a disease in which ICB has proven efficacy. Beyond HLA–TCR interactions, other types of cellular contact between TFH cells and LP cells, and the nature of the contact between LP cells and the other T-cell subtypes that rosetted and how these are influenced by immune-checkpoint expression also remains to be resolved.
Unlike melanoma (where PD-L1 amplification is only <1%),61, 62 an important component of ICB success in cHL is due to copy number gains and amplifications of PD-L1/PD-L2 in HRS cells, with the EBV also contributing to increased expression.17, 63 Research is ongoing to better understand the role of PD-L1/PD-L2 as a lymphoma immune-oncology marker.64-66 In contrast to cHL, amplifications of the 9p.24.1 locus occur less frequently in NLPHL,67 with conflicting results in the literature regarding the frequency of PD-L1 expression on LP cells.68, 69 Expression might occur as a result of upstream JAK signaling in LP cells due to mutations of SOCS1.70, 71 We observed lower PD-L1 gene levels in NLPHL relative to cHL and this was confirmed at a protein level by multiplexed tissue-microarray. However, PD-L2 gene levels in cHL were equivalent to NLPHL. Interestingly, the NLPHL cell-line DEV contains a PD-L2 fusion-transcript and high PD-L2 protein.19 Finally, CD155 (a ligand for TIGIT) was expressed on the cell surface of LP and HRS cells, with levels lower in NLPHL. In the absence of clinical trials, it remains untested if immune-checkpoint ligands are predictive biomarkers of ICB efficacy in NLPHL.
Studies investigating dual PD-1/TIGIT ICB in patients with advanced solid tumors show promising anti-tumoral activity.72, 73 Moreover, TIGIT is abundantly expressed on Tregs.74, 75 Hence dual-blockade of PD-1/TIGIT may shift the TME to a more anti-tumoral state. Blockade of other immune-checkpoints may also be of benefit.76 Dual-blockade of PD-1/TIGIT in NLPHL could be combined with bi-specific T-cell engagers targeting B-cell antigens. Although combinatorial ICB targeting offers potential benefit, clinical studies must be undertaken cautiously, as enhanced efficacy might be offset by increased immune-related adverse events.
Recent data indicates selected NLPHL patients might be appropriately treated in a less aggressive manner.77 However, excluding anti-CD20 antibodies, no “chemo-free” systemic therapies are available.78 PD-1 blockade has efficacy in cHL. Here, we show that compared to cHL, the TME of NLPHL is enriched in PD-1 and TIGIT. Overall, our results indicate that PD-1 targeting, perhaps in combination with TIGIT blockade, warrants further evaluation in NLPHL.
AUTHOR CONTRIBUTIONS
M.K.G. and J.G. conceived the project and together with S.C.L. designed and performed the research, analyzed and interpreted data, and co-wrote the manuscript. M.B.S., É.F., A.H., C.I. L., P.G.M, K.B., A.Z., J.N. L., S. B (Brosda), S.B (Birch), C. S (Snell), and M.B. (Burgess) performed experiments and interpreted data. J.T., L.B., E. J., E. A. H., K. N., F. S., J. W. D. T., D. T., S. J., E.B., M. S., C.S. (Steidl), K.S., P.F., M.B. (Boyle)., B.M., M.R.G., F.V., and C.K. curated the study cohort and interpreted data. All authors approved the final version.
ACKNOWLEDGMENTS
Work in the laboratory of M.K.G. is supported by the Mater Foundation. J.G. is supported by an American Society of Hematology and a Cancer Australia Priority-driven Collaborative Cancer Research Scheme (Leukaemia Foundation) grant. The Australasian Leukaemia Lymphoma Group provided cHL samples from RATHL supported by an Australian National Health and Medical Research Council grant awarded to M.K.G. J.T., and L.B. We thank histology, microscopy, and flow cytometry core facilities at the Translational Research Institute for technical support, and ACT Haematology Research Tissue Bank for provision of their samples. FV is supported by a R01CA222918 from the National Cancer Institute. É.F. and C.I.L. are supported by Irish Research Council Grants. É.F. and A.H. are partly funded by the Limerick Digital Cancer Research Centre. MRG is supported by a Leukemia and Lymphoma Society Scholar Award. Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
E.A.H: Research funding to institution; Bristol Myers Squibb, Merck KgA, Astra Zeneca, Roche, Advisory board: Roche*, Antigene*, Bristol Myers Squibb, Astra Zeneca, Novartis*, Merck Sharpe Dohme*, Gilead*, Beigene* (*paid to institution); Speakers fees: Roche*, Astra Zeneca*, Abbvie*, Janssen, Regeneron, (*paid to institution); Consultancy: Regeneron, Bristol Myers Squibb, Specialized therapeutics. D.T: research funding from Roche, Janssen and Beigene, and honoraria from CSL, Antengene, Roche, Beigene, Amgen, and EUSA Pharma. M.K.G.: honoraria from Novartis, Gilead. F.V. receives research funding from Caribou, Allogene, Geron corporation, and received in the last 3 years honoraria from Oakstone Medical Publishing, i3Health, Elsevier, America Registry of Pathology, Congressionally Directed Medical Research Program, Society of Hematology Oncology and National Research Foundation of Singapore (28th Competitive Research Program Whitepapers). E.B: consultancy Abbvie, Astellas, Gilead, IQVIA, MSD, Novartis and Bastion Brands, Research funding MSD. MRG: research funding from Sanofi, Kite/Gilead, Abbvie and Allogene; consulting for Abbvie, Allogene and Bristol Myers Squibb; honoraria from BMS, Daiichi Sankyo and DAVA Oncology; and stock ownership of KDAc Therapeutics.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the Data S1 of this article.




