Impact of busulfan versus treosulfan dose intensity in myelofibrosis undergoing hematopoietic cell transplantation
Abstract
One key aspect of allogeneic hematopoietic cell transplantation (HCT) is pretransplant conditioning, balancing risk for relapse versus non-relapse mortality. Conditioning regimens with different alkylators at different doses can influence outcome, but data are missing for myelofibrosis, a challenging cohort of patients usually presenting at older age and with comorbidities. We evaluated in a multicenter retrospective study the comparative efficacy and safety of busulfan versus treosulfan in combination with fludarabine for myelofibrosis patients undergoing HCT. This study included 1115 patients (busulfan, n = 902; treosulfan, n = 213) receiving first HCT between 2005 and 2021. Patients were generally balanced for key patient characteristics. Overall survival at 4 years was 62% for the busulfan group versus 58% for the treosulfan group (p = .22). Impact on outcome was dose-dependent. Overall survival was 65% (95% CI, 61%–69%) for reduced intensity busulfan versus 69% (95% CI, 54%–84%) for reduced intensity treosulfan, 53% (95% CI, 44%–63%) for higher intensity busulfan, and 55% (95% CI, 46%–63%) for higher intensity treosulfan. Incidence of relapse was similar across intensity groups. In multivariable analysis, the hazard for death (with reduced intensity busulfan as reference) was 0.88 (95% CI, 0.39–2.01) for reduced intensity treosulfan (p = .77), 1.42 (95% CI, 0.96–2.10) for higher intensity busulfan (0.08), and 1.61 (95% CI, 1.14–2.26) for higher intensity treosulfan (p = .006). In terms of non-relapse mortality, comparison was not significantly different, while the hazard ratio for higher intensity treosulfan was 1.48 (95% CI, 0.98–2.23; p = .06). Here, we showed comparable outcomes and improved survival in myelofibrosis undergoing HCT with reduced intensity busulfan or treosulfan.
1 INTRODUCTION
Myelofibrosis is a chronic myeloproliferative neoplasm arising either de novo (primary, PMF) or secondary (SMF) evolving from essential thrombocytosis or polycythemia vera. Allogeneic hematopoietic cell transplantation (HCT) is the only potentially curative option for eligible patients.1 One key aspect of HCT is pretransplant conditioning, balancing risk for relapse and risk for non-relapse mortality due to, for instance, infections or graft-versus-host disease.2 Myelofibrosis patients usually present at older age and with comorbidities, challenging intensive treatment in this population.
The recognition of the substantial association of a graft-versus-tumor effect and the high toxicity of the commonly used conditioning regimen led to the introduction of more differentiated intensity strategies, with the aim of making hematopoietic stem-cell transplantation less toxic and safer, and thus more applicable to broader populations such as older or unfit patients. However, comparative studies of reduced versus higher intensity conditioning, across regimen, showed similar outcomes in patients with myelofibrosis.3, 4 Other studies suggested that reduced intensity conditioning using busulfan and fludarabine, the most widely used regimen, showed best outcomes.5, 6 More recently, reduced toxicity regimen using another alkylator treosulfan in combination with fludarabine, exhibiting lower inter- and intra-patient variability without the need for dose adjustments,7 was shown to improve outcomes in older and more frail patients with acute myeloid leukemia.8 Early studies investigating treosulfan and fludarabine based conditioning indicated potent myeloablative and anti-myelofibrosis activity although non-relapse mortality remained high.9 In addition, the combination of treosulfan and fludarabine together with thymoglobulin has been shown to result in promising outcomes for patients with relapsed myelofibrosis, where relapse treatment such as donor lymphocyte infusions failed.10, 11
In light of lacking evidence for a comparison of both alkylators in patients with myelofibrosis, we evaluated the comparative efficacy and safety of busulfan versus treosulfan in combination with fludarabine for myelofibrosis patients undergoing HCT.
2 METHODS
2.1 Data collection
We included a population of myelofibrosis patients undergoing first HCT from 2005 until 2021 receiving either busulfan or treosulfan in combination with fludarabine for conditioning therapy prior to HCT, and who have been reported to the German registry for stem cell transplantation (Deutsches Register für Stammzelltransplantation). The choice and reasons for a specific regimen was up to the physician's discretion. Disease risk for myelofibrosis at time of HCT was calculated according to the Dynamic International Prognostic Scoring System (DIPSS).12 Patients with transformed acute leukemia at the time of HCT have been excluded. Detailed information on conditioning dose, graft-versus-host disease prophylaxis, comorbidity status, performance status, donor characteristics including HLA-match, donor sex and age, diagnosis at the time of HCT (PMF or SMF), driver mutation genotype (JAK2, CALR, or MPL), and other patient-specific variables were collected. For comorbidities, the HCT comorbidity index (HCT-CI) was calculated and categorized as previously reported.13 Informed consent had been retrieved prior to reporting. This study is in accordance with the Declaration of Helsinki.
2.2 Endpoints and definitions
The primary objective of this study was to evaluate the comparative efficacy and safety of busulfan versus treosulfan in combination with fludarabine for first HCT in patients with myelofibrosis. Endpoints of interest were overall survival, non-relapse mortality, relapse incidence, engraftment, and incidence of acute and chronic graft-versus-host disease. Overall survival was defined as death from any cause. Non-relapse mortality and incidence of relapse were considered as competing events. Neutrophil engraftment was defined as the first of three consecutive days of achieving a sustained peripheral blood neutrophil count of >0.5 × 109/L, and platelet engraftment was defined as independence from platelet transfusion for at least 7 days with a platelet count of more than >20 × 109/L.
Conditioning intensity was stratified into reduced and higher intensity therapy, in line with previously described studies.6, 14 Transplants with total dose of up to 8 mg/kg orally or 6.4 mg/kg intravenously of busulfan or 30 g/m2 of treosulfan were considered reduced intensity HCT, while the remaining higher doses were considered higher intensity HCT.
2.3 Statistical analysis
This is a retrospective cohort study comparing outcomes after busulfan versus treosulfan conditioning in combination with fludarabine before HCT for patients with myelofibrosis. Categorical variables between the groups were compared using the Chi-squared test, and continuous variables were compared using the Mann–Whitney test.
Probabilities of survival were calculated using Kaplan–Meier estimates. Probabilities of non-relapse mortality and relapse were calculated by cumulative incidence function (Nelson–Aalen estimator) accounting for competing risks. Cause-specific hazard ratios (HR) were obtained for univariable analysis of potentially confounding factors for the main comparison of busulfan versus treosuflan. Multivariable analyses were performed to evaluate the independent effect of type of conditioning together with associations among other patient-related, disease-related, donor-related, and transplantation-related variables. Cox proportional hazards regression model was used for survival and Fine and Gray modeling for competing risk outcomes. Backward stepwise selection (using best Akaike information criterion) was used to identify significant covariates that influenced outcomes of busulfan versus treosulfan. Comparisons with p < .05 were considered significantly different. The proportional hazards assumption for Cox regression was tested using Schoenfeld residuals. In addition, a Dependent Dirichlet Process model for survival analysis data was developed. A major feature of the proposed approach is that there is no necessity for resulting survival curve estimates to satisfy the ubiquitous proportional hazards assumption. In case of missing information, multiple imputation was used. All analyses were performed with R statistical software version 4.0.5.
3 RESULTS
3.1 Patients
This study included 1115 patients (busulfan, n = 902; treosulfan, n = 213) receiving first HCT between 2005 and 2021 (Figure S1). The median year of HCT was 2015 for busulfan and 2017 for treosulfan. Patient characteristics were generally balanced (Table 1), showing similar distribution according to performance status, HCT-CI, diagnosis, driver mutation genotype, and donor age. Median age at HCT was similar between both groups (59 years, respectively), while more patients in the treosulfan group were 65 years or older (32% vs. 26%, p = .03). Distribution of patient and transplant characteristics was similar when excluding patients receiving HCT before 2015. Disease categorization according to DIPSS was available in approximately half of the patients, being intermediate-1 risk in 32% for busulfan and 45% for treosulfan, intermediate-2 risk in 45% and 30%, and high risk in 23% and 24%, respectively (p = .007).
| Characteristics | Busulfan-fludarabine (n = 902) | Treosulfan-fludarabine (n = 213) | p |
|---|---|---|---|
| Age, median (range) | 59 (22–78) | 59 (37–76) | .38 |
| Age category, no. (%) | .03 | ||
| <65 years | 670 (74) | 144 (68) | |
| ≥65 years | 232 (26) | 69 (32) | |
| Sex, no. (%) | .44 | ||
| Male | 557 (62) | 122 (57) | |
| Female | 345 (38) | 91 (43) | |
| Diagnosis, no. (%) | .15 | ||
| Primary myelofibrosis | 655 (73) | 165 (78) | |
| Secondary myelofibrosis | 247 (27) | 48 (22) | |
| DIPSS category, no. (%) | |||
| Intermediate-1 | 156 (32) | 52 (45) | |
| Intermediate-2 | 224 (45) | 35 (30) | |
| High | 112 (23) | 28 (24) | |
| HCT-CI, no. (%) | .47 | ||
| 0 | 332 (47) | 80 (44) | |
| 1–2 | 202 (29) | 51 (28) | |
| ≥3 | 166 (24) | 51 (28) | |
| Karnofsky performance status, no. (%) | .74 | ||
| 90%–100% | 565 (66) | 128 (65) | |
| <90% | 292 (34) | 70 (35) | |
| Driver mutation genotype, no. (%) | .80 | ||
| CALR | 86 (14) | 21 (14) | |
| JAK2 | 446 (75) | 107 (73) | |
| MPL | 28 (5) | 6 (4) | |
| HLA-match, no. (%) | .07 | ||
| Identical sibling | 203 (23) | 40 (19) | |
| Matched unrelated | 566 (63) | 146 (69) | |
| Haploidentical | 3 (<1) | 3 (1) | |
| Mismatched unrelated | 130 (14) | 24 (11) | |
| Use of ATG, no. (%) | 899 (99.7) | 206 (96.7) | <.001 |
| Median dose of alkylator | 8 mg/kg | 32 g/m2 | |
| Donor age, median (range) | 35 (17–72) | 35 (19–76) | .50 |
| Donor sex, no. (%) | .07 | ||
| Male | 652 (72) | 161 (75) | |
| Female | 255 (28) | 52 (24) | |
| Transplant year, median (range) | 2015 (2005–2021) | 2017 (2005–2021) | <.001 |
- Abbreviations: DIPSS, Dynamic International Prognostic Scoring System; HCT-CI, hematopoietic cell transplant comorbidity index.
The median dose of conditioning was 8 mg/kg for the busulfan group and 42 g/m2 for the treosulfan group. In terms of conditioning intensity, 84% of patients (n = 718) in the busulfan group received reduced intensity dose while 22% of patients (n = 46) in the treosulfan group received reduced intensity dose. Patient characteristic distribution was similar when comparing both groups according to reduced or higher intensity dose (Table S1).
3.2 Outcomes of busulfan versus treosulfan
The median follow-up was comparable between both the groups, being 4.3 years (95% CI, 3.9–4.7 years) for the busulfan group and 3.9 years (95% CI, 2.8–5.0 years) for the treosulfan group. Likelihood of achieving neutrophil engraftment was higher in the busulfan group versus the treosulfan group, being 96% versus 92% at a median of 18 days (p = .09), respectively (Figure S2). The rates of primary graft failure were 4% in the busulfan group and 8% in the treosulfan group, with similar rates across intensities. Platelet engraftment occurred in 89% of patients in the busulfan group and 84% in the treosulfan group (p = .07), for both at a median of 20 days.
Overall survival at 4 years was 62% (95% CI, 58%–65%) for the busulfan group versus 58% (95% CI, 50%–65%) for the treosulfan group (Figure S3). Relapse incidence at 1 year was 20% (95% CI, 17%–22%) for the busulfan group versus 20% (95% CI, 14%–25%) for the treosulfan group and non-relapse mortality at 1 year was 21% (95% CI, 19%–24%) versus 19% (95% CI, 14%–24%). Progression-free survival at 4 years was 50% (95% CI, 47%–54%) for the busulfan group versus 48% (95% CI, 40%–55%) for the treosulfan group.
3.3 Impact of dose intensity
Comparison of conditioning regimen and dose intensity showed a significant interaction for overall outcomes (p < .001; Figure 1). The median follow-up was also significantly different between the four dose-intensity groups, due to longer follow-up for patients receiving higher intensity busulfan (median, 6 years) compared with reduced intensity busulfan (median, 4.1 years), higher intensity treosulfan (median, 3.9 years), and lower intensity treosulfan (median, median 4.2 years).
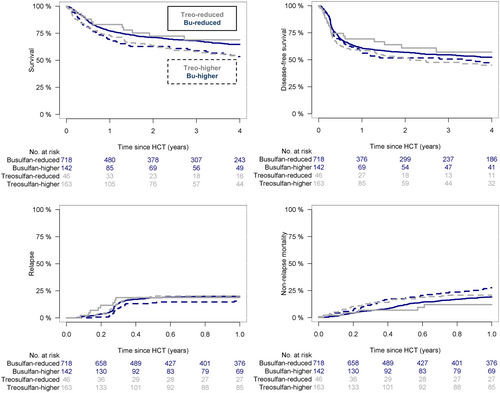
Overall survival at 4 years was 65% (95% CI, 61%–69%) for reduced intensity busulfan versus 69% (95% CI, 54%–84%) for reduced intensity treosulfan. Higher intensity doses appeared to be associated with worse overall survival, being 53% (95% CI, 44%–63%) for higher intensity busulfan and 55% (95% CI, 46%–63%) for higher intensity treosulfan. Pairwise comparisons showed significantly worse survival with higher intensity treosulfan (p = .03). Progression-free survival at 4 years was 52% (95% CI, 48%–56%) for reduced intensity busulfan versus 57% (95% CI, 41%–73%) for reduced intensity treosulfan versus 46% (95% CI, 37%–55%) for higher intensity busulfan versus 45% (95% CI, 36%–54%) for higher intensity treosulfan. Pairwise comparisons were not statistically significant. Relapse incidence at 1 year was 20% (95% CI, 17%–23%) for reduced intensity busulfan versus 19% (95% CI, 7%–30%) for reduced intensity treosulfan versus 16% (95% CI, 19%–22%) for higher intensity busulfan versus 20% (95% CI, 14%–27%) for higher intensity treosulfan, and non-relapse mortality was 19% (95% CI, 16%–22%) for reduced intensity busulfan versus 12% (95% CI, 2%–22%) for reduced intensity treosulfan versus 28% (95% CI, 20%–35%) for higher intensity busulfan versus 21% (95% CI, 14%–27%) for higher intensity treosulfan, respectively. Pairwise comparisons for relapse and non-relapse mortality showed no significant difference between reduced intensity busulfan and treosulfan. Compared with higher intensity busulfan dose schedules, reduced intensity busulfan appeared to be associated with lower non-relapse mortality (Log rank p = .08). The remaining comparisons were not statistically significant.
3.4 Impact of HCT year
To account for significantly different distribution of both conditioning strategies according to HCT year (Table 1 and Figure S4), we evaluated outcomes for patients transplanted before 2015 and between 2015 and 2021, showing similar overall survival for the total cohort, being 61% (95% CI, 56%–65%) before 2015 and 62% (95% CI, 57%–66%) between 2015 and 2021. However, non-relapse mortality appeared to be slightly decreased for HCT between 2015 and 2021, showing incidence at 1 year of 19% (95% 15%–22%) versus 24% (95% CI, 20%–27%) for patients transplanted before 2015, while relapse incidence was stable showing 1-year cumulative incidence of 20% (95% CI, 16%–24%) for HCT before 2015 and 19% (95% CI, 16%–22%) for HCT between 2015 and 2021.
3.5 Factors on outcome
Univariable analysis with cause-specific hazards investigated a potential role of factors on main objective of overall survival. No significant difference was found for the general comparison of busulfan versus treosulfan (p = .22). Accounting for the interaction of regimen and dose intensity, cause-specific hazard (with reduced intensity busulfan as reference) was 0.97 (95% CI, 0.58–1.61) for reduced intensity treosulfan, 1.28 (95% CI, 0.97–1.69) for higher intensity busulfan, and 1.35 (95% CI, 1.04–1.77) for higher intensity treosulfan. Significant association with overall survival was identified for patient age, driver mutation genotype, HLA-match, and performance status, and univariable association with worse non-relapse mortality was shown for older patient age, high HCT-CI, poor performance status, mismatched unrelated donor HCT, and transplants before 2015 (Table 2).
| Overall survival | Non-relapse mortality | |||||
|---|---|---|---|---|---|---|
| Factor | Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p |
| Regimen | ||||||
| Busulfan | Reference | |||||
| Treosulfan | 1.16 | 0.92–1.47 | .22 | 1.17 | 0.81–1.68 | .40 |
| Dose intensity | ||||||
| Busulfan-reduced | Reference | |||||
| Busulfan-higher | 1.28 | 0.97–1.69 | .08 | 1.32 | 0.84–2.07 | .23 |
| Treosulfan-reduced | 0.97 | 0.58–1.61 | .90 | 0.73 | 0.28–.94 | .53 |
| Treosulfan-higher | 1.35 | 1.04–1.77 | .03 | 1.33 | 0.90–1.97 | .15 |
| Age, continuous | 1.03 | 1.02–1.05 | <.001 | 1.04 | 1.02–1.06 | <.001 |
| Age category | ||||||
| <65 years | Reference | |||||
| ≥65 years | 1.46 | 1.19–1.79 | <.001 | 1.69 | 1.24–2.29 | <.001 |
| Sex | ||||||
| Female | Reference | |||||
| Male | 1.18 | 0.97–1.43 | .11 | 1.11 | 0.90–1.36 | .35 |
| Diagnosis | ||||||
| Primary myelofibrosis | Reference | |||||
| Secondary myelofibrosis | 0.96 | 0.77–1.19 | .72 | 0.84 | 0.60–1.18 | .31 |
| HCT-CI | ||||||
| 0 | Reference | |||||
| 1–2 | 1.14 | 0.87–1.49 | .34 | 1.34 | 0.95–1.89 | .10 |
| ≥3 | 1.51 | 1.15–1.99 | .003 | 1.50 | 1.03–2.17 | .03 |
| Karnofsky performance status | ||||||
| 90%–100% | Reference | |||||
| <90% | 1.69 | 1.38–2.07 | <.001 | 1.61 | 1.30–1.99 | <.001 |
| DIPSS category | ||||||
| Intermediate-1 | Reference | |||||
| Intermediate-2 | 0.90 | 0.65–1.24 | .51 | 0.88 | 0.63–1.21 | |
| High | 1.53 | 1.08–2.17 | .02 | |||
| Driver mutation genotype | ||||||
| CALR | Reference | |||||
| JAK2 | 1.60 | 1.07–2.39 | .02 | 1.35 | 0.86–2.13 | .19 |
| MPL | 0.84 | 0.37–1.93 | .69 | 0.75 | 0.28–1.99 | .56 |
| HLA-match | 1.38 | 0.70–2.71 | .36 | |||
| Identical sibling | Reference | |||||
| Matched unrelated | 1.34 | 1.04–1.72 | .02 | 1.07 | 0.72–1.59 | .73 |
| Haploidentical | 0.77 | 0.11–5.57 | .80 | 1.33 | 0.24–7.36 | .74 |
| Mismatched unrelated | 1.76 | 1.28–2.41 | <.001 | 1.86 | 1.16–3.00 | .01 |
| Donor age, continuous | 1.00 | 0.99–1.01 | .86 | 1.00 | 0.99–1.01 | .96 |
| Donor sex | ||||||
| Female | Reference | |||||
| Male | 0.82 | 0.61–1.11 | .20 | 0.85 | 0.64–1.14 | .32 |
| HCT year, continuous | 1.00 | 0.97–1.02 | .71 | 0.97 | 0.94–1.01 | .12 |
| HCT year category | ||||||
| <2015 | Reference | |||||
| 2015–2021 | 0.98 | 0.80–1.20 | .86 | 0.71 | 0.50–1.00 | .05 |
- Abbreviations: CI, confidence interval; DIPSS, Dynamic International Prognostic Scoring System; HCT-CI, hematopoietic cell transplant comorbidity index.
3.6 Multivariable model
To account for potential confounders and evaluate independent effects on outcome, a multivariable model was developed (Figure 2). For the comparison of busulfan and treosulfan on overall survival and according to different dose intensities, the hazard for death (with reduced intensity busulfan as reference) was 0.88 (95% CI, 0.39–2.01) for reduced intensity treosulfan (p = .77), 1.42 (95% CI, 0.96–2.10) for higher intensity busulfan (0.08), and 1.61 (95% CI, 1.14–2.26) for higher intensity treosulfan (p = .006). Other independent factors for worse overall survival were JAK2 driver mutation genotype (with CALR driver mutation genotype as reference), mismatched unrelated donor HCT (with identical sibling HCT as reference), and performance status <90% (with a Karnofsky performance status 90%–100% as reference).
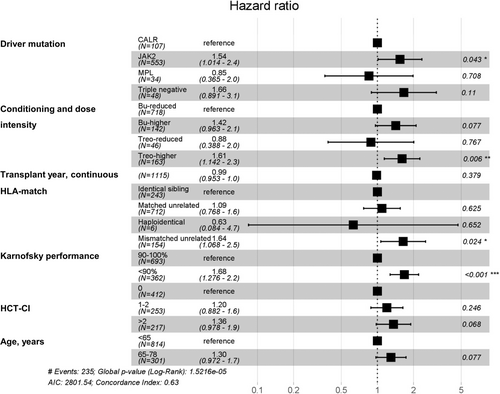
In terms of non-relapse mortality, hazard for death (with relapse as competing event and reduced intensity busulfan as reference) appeared to be significantly higher only for higher intensity treosulfan. Other independent predictors for worse outcome appeared to be older patient age, mismatched unrelated donor HCT, poor performance status, and transplants before 2015 (Table S2).
4 DISCUSSION
To the best of our knowledge, this is the largest study comparing busulfan and treosulfan conditioning for patients with myelofibrosis undergoing first HCT. We showed for the first time that outcomes were dose-dependent for both regimens, with comparable outcomes for both reduced busulfan and treosulfan intensity strategies, which furthermore appeared to be better than higher intensity strategies. Strength of this analysis is the comparability of treatment groups for key patient characteristics, including age, comorbidities, and performance status.
In fact, more than half of patients in both treatment groups had an HCT-CI of more than zero. Our results support reduced intensity rather than higher intensity treosulfan HCT in such challenging patient cohorts and are in line with the pivotal prospective trial by Beelen et al.14, 15 comparing treosulfan with busulfan in older patients with acute myeloid leukemia and myelodysplastic syndromes, which was initially registered with a treosulfan dose of 14 g/m2 daily on Days −6 to −4 of the 6-day regimen and subsequently modified the trial protocol using the dose to 10 g/m2 treosulfan daily applied as a 2-h infusion for 3 days (Days −4 to −2) after safety concerns. In addition, a first study in myelofibrosis by Claudiani et al. similarly showed, although with promising myelablative capability, higher intensity treosulfan was associated with high non-relapse mortality.9 In our cohort, higher intensity treosulfan was the only conditioning strategy that appeared to be significantly associated with higher risk for non-relapse mortality.
In contrast to the prospective trial, which did not include patients with myelofibrosis, we did not find a significant difference in outcome between reduced intensity busulfan and treosulfan HCT. A recent retrospective registry study by the European Society for Blood and Marrow Transplantation (EBMT) suggested that treosulfan based regimens compared to busulfan based regimens showed better disease-free survival in in myelofibrosis, which was confirmed for overall survival and non-relapse mortality when comparing (reduced together with higher intensity) treosulfan versus higher intensity busulfan conditioning. While survival results of the treosulfan cohort in that study resemble the results shown in our study for reduced intensity treosulfan, the main difference between our analysis and the EBMT study was the outcome of reduced intensity busulfan, which appeared to be relatively low in the EBMT study showing 3-year overall survival of only 60% versus 68% (and 65% at 4 years) in the present analysis. This difference appears to be driven by lower non-relapse mortality in the reduced intensity busulfan group in our analysis which was similar to reduced intensity treosulfan as well as the treosulfan arm in the EBMT study (which did not account for different dose intensities and included significantly lower number of patients, n = 74).16
Three large comparisons evaluating the role of conditioning intensities and regimens have recently been reported in patients with myelofibrosis. First, in a previous analysis from the EBMT of more than 2000 patients receiving HCT between 2000 and 2014,4 65% received reduced intensity and 35% myeloablative conditioning. Half of the patients in each group received busulfan-fludarabine conditioning. Median follow-up time was similar compared to ours, while overall outcome in this study was similar for both intensities, although absolute relapse rates appeared to be higher for reduced intensity conditioning, being 23% at 5 years, which was slightly higher compared with our results. Second, a recent study from the Center for International Blood and Marrow Transplant Research database suggested that fludarabine-busulfan was associated with better outcomes in reduced intensity HCT (showing better overall survival and lower early non-relapse mortality) as well as in higher intensity conditioning (showing better GVHD-/relapse-free survival).17 A crude comparison of busulfan-fludarabine reduced versus higher intensity in this study suggested better outcomes for reduced intensity busulfan-fludarabine conditioning. Third, an international analysis evaluated the value of higher intensity conditioning in the era of molecular risk stratification in myelofibrosis, finding no difference in outcome for patients with reduced or higher intensity conditioning and high molecular risk.3, 18, 19 This study included mostly busulfan-fludarabine for reduced and busulfan-cyclophosphamide for higher intensity conditioning. Of note, none of the studies reported outcomes for treosulfan based conditioning. In addition, in our analysis, we included only busulfan-fludarabine or treosulfan-fludarabine strategies for reduced or higher intensity conditioning, respectively, suggesting better overall survival for both reduced intensity versus higher intensity strategies. Overall, our results are in line with the report from the CIBMTR, with the important addition of reduced treosulfan conditioning providing comparable outcome to busulfan, while higher intensity conditioning for both groups appeared to be associated with worse survival and higher intensity treosulfan conditioning was associated with higher non-relapse mortality. Key difference to the EBMT studies could be the introduction of heterogeneity and potential for overinterpretation of small samples when retrospectively including outcomes across countries and practices, while the CIBMTR and our study rather include homogenous cohorts.
Our results underscore the relevance of minimizing toxicity in patients with myelofibrosis, who are mostly older and comorbid. Patients in our analysis were well balanced for the HCT-CI. However, this score appears to have less value in the modern era where patient selection and surveillance have been improved significantly compared to 2005 when the HCT-CI was introduced.20, 21 A recent publication from EBMT suggests value of the HCT-CI in patients with myelofibrosis.22 However, detailed results on specific comorbidities were lacking. Rather, outcome appears to be associated with regimen-specific profiles, suggesting increased non-relapse mortality for busulfan-fludarabine in patients with cardiac disease, and for treosulfan-fludarabine in patients with severe pulmonary disease and a preexisting infection in a recent study.23
Additionally, while more patients in the treosulfan group and reduced intensity busulfan group were transplanted more recently compared with higher intensity busulfan based HCT, our analysis indicated a slightly decreased incidence of non-relapse mortality for HCT from 2015 compared with HCT given before 2015, with a stable relapse incidence (~20% at 1 year after HCT). Digging deeper into potential factors for this finding opened a contrasting landscape. Patients transplanted between 2015 and 2021 were more likely to present with higher HCT-CI and worse performance status, whereas number of transplants using mismatched unrelated donors decreased significantly from 2015. Overall, whether the slight decrease in mortality is therefore associated with improved patient and donor selection or is influenced by introduction of novel agents is still unclear and beyond the scope of the current study but should be addressed in future studies for all HCT strategies and regimens.
We acknowledge several limitations, mainly due to the retrospective nature of the study. The comparison between reduced intensity strategies using busulfan or treosulfan is limited by the relatively small sample size of patients receiving reduced intensity treosulfan. We cannot account for lack of transparency for physician choice of a specific regimen and its intensity. Furthermore, we did not have sufficient information on pharmacokinetics of busulfan monitoring and whether targeted dosing of busulfan influenced outcomes. Of note, we found no significant differences for several important patient characteristics and applied multivariable modeling to adjust for potential confounders. Furthermore, incomplete data on the patient level especially for novel data including mutation status beyond driver mutation genotype do not allow comparison of regimen and intensity for a specific mutational risk, including high molecular risk (including ASXL1, EZH2, SRSF2, IDH1/2, or TP53).19, 24, 25
In conclusion, we showed for the first time comparable outcomes in patients with myelofibrosis undergoing first HCT with reduced intensity busulfan or treosulfan conditioning in combination with fludarabine. The impact on outcome was dose-dependent showing better outcomes for both reduced intensity strategies compared with higher intensity strategies, with higher intensity treosulfan-fludarabine being associated with significantly higher non-relapse mortality while both higher intensity strategies (busulfan or treosulfan) were associated with significantly reduced overall survival.
AUTHOR CONTRIBUTIONS
NG and NK designed the study, analyzed and interpreted data, wrote the first draft of manuscript. CS collected the data. SF gave statistical input. DK, MS, IWB, AB, WB, TS, GW, ES, KF, and GS interpreted data and wrote the manuscript. All authors approve of the final version of the manuscript.
ACKNOWLEDGMENT
Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
ES received honoraria for consulting activities or travel support from Gilead, Jazz, MSD, Neovii, Novartis, Priothera, Medac, and Therakos.
PATIENT CONSENT
Each patient gave their consent to use their data for academic purposes either based on DRST documents or center-specific consent statements.
Open Research
DATA AVAILABILITY STATEMENT
Original data can be retrieved from the corresponding author upon reasonable request.




