Cost-effectiveness of sutimlimab in cold agglutinin disease
Satoko Ito and Daniel Wang are co-first authors.
Abstract
Primary cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia caused by cold-reactive antibodies that bind to red blood cells and lead to complement-mediated hemolysis. Patients with primary CAD experience the burden of increased health resource utilization and reduced quality of life. The standard-of-care (SOC) in patients with primary CAD has included cold avoidance, transfusion support, and chemoimmunotherapy. The use of sutimlimab, a humanized monoclonal antibody that selectively inhibits C1-mediated hemolysis, was shown to reduce transfusion-dependence and improve quality of life across two pivotal phase 3 studies, further supported by 2-year extension data. Using data from the transfusion-dependent patient population that led to sutimlimab's initial FDA approval, we performed the first-ever cost-effectiveness analysis in primary CAD. The projected incremental cost-effectiveness ratio (ICER) in our Markov model was $2 340 000/QALY, significantly above an upper-end conventional US willingness-to-pay threshold of $150 000/QALY. These results are consistent across scenarios of higher body weight and a pan-refractory SOC patient phenotype (i.e., treated sequentially with bendamustine-rituximab, bortezomib, ibrutinib, and eculizumab). No parameter variations in deterministic sensitivity analyses changed our conclusion. In probabilistic sensitivity analysis, SOC was favored over sutimlimab in 100% of 10 000 iterations. Exploratory threshold analyses showed that significant price reduction (>80%) or time-limited treatment (<18 months) followed by lifelong clinical remission off sutimlimab would allow sutimlimab to become cost-effective. The impact of sutimlimab on health system costs with longer term follow-up data merits future study and consideration through a distributional cost-effectiveness framework.
1 INTRODUCTION
Primary cold agglutinin disease (CAD) is a subtype of autoimmune hemolytic anemia in which red blood cells (RBCs) are bound by cold agglutinins—autoantibodies which react optimally at temperatures of 3–4°C—leading to complement-mediated extravascular hemolysis.1-3 Primary CAD is distinguished from cold agglutinin syndrome, which is transient and self-remitting condition triggered by various underlying disorders such as infections, malignancy, and autoimmune disease.4, 5 Some patients with CAD experience severe, transfusion-dependent anemia requiring multiple lines of therapy.2, 3 CAD generally affects older populations, with risk of onset increasing after the age of 55 and median age of 65 found in a US-based population study.6 Health resource utilization is significantly higher for patients with CAD compared to age- and sex-matched controls, and quality of life studies have found a significant burden of disease with up to 90% of patients reporting fatigue on a daily basis.2, 7-9 The standard-of-care (SOC) for unselected primary CAD includes non-pharmacologic and supportive care, including cold avoidance and RBC transfusions, as needed.10 Despite these measures, up to 70% of patients require some form of pharmacologic intervention.3, 11 These treatments have historically targeted pathogenic B cell clones responsible for producing cold agglutinins, with agents such as rituximab, fludarabine, and bendamustine showing efficacy.7, 10, 12, 13
Recent advancements have seen the development of targeted complement inhibitors for a variety of hematologic conditions. For primary CAD this includes the humanized monoclonal antibody sutimlimab, which selectively inhibits C1s-mediated hemolysis. Sutimlimab is currently the only FDA-approved therapeutic for patients with CAD regardless of history of transfusion, with approval based on the CARDINAL and CADENZA phase 3 clinical studies.14, 15 However, the cost-effectiveness of this expensive new therapeutic in the care of patients with either transfusion-independent or transfusion-dependent primary CAD is not known. We sought to fill this gap by determining the cost-effectiveness of sutimlimab versus the standard-of-care for transfusion-dependent patients with primary CAD.
2 METHODS
2.1 Model overview
We developed a Markov simulation model to estimate the cost-effectiveness of sutimlimab compared to SOC in patients with transfusion-dependent primary CAD (phase 3 CARDINAL study), a patient population that incurs greater risks and costs as compared to transfusion-independent primary CAD (phase 3 CADENZA study).14, 15 We defined patients with transfusion-dependent primary CAD as patients who had confirmed CAD and needed red blood cell transfusion within the past 6 months, according to the inclusion criteria of the CARDINAL study.15 Our model was anchored in data from the phase 3, open-label, non-randomized study based on which the FDA initially approved sutimlimab in 2022 (CARDINAL, part A): (1) SOC baseline inclusive of (i) 6-month pre-enrollment, (ii) 6-week screening period and (2) 6-month study treatment period.15 Of note, recent 2-year extension studies have shown sustainment in both efficacy and quality of life in patients with transfusion-dependent primary CAD while on therapy with sutimlimab, returning to baseline following treatment cessation.15-17 Patients enrolled in this study required an average of 3.2 transfusions in the 6-month enrollment period while receiving SOC. A total of 13 (54%) patients achieved improvement in hemoglobin without transfusions at 26 weeks, and 71% of patients remained transfusion-free from weeks 5 to 26. Significant fatigue reduction was observed by week 1 and continued throughout the study period. In our model, patients with transfusion-dependent primary CAD were assigned to one of two treatment arms: sutimlimab or SOC. Patient characteristics were informed by the CARDINAL study and consistent in age and sex distribution of a typical CAD population.6, 7, 18 Patients entered the model at a median age of 72 years with 62% being female. Our model consists of health states that mirror the clinical trial focused on transfusion-independence and transfusion dependence (in addition to the background probability of mortality, especially important in the lifetime base-case), allowing for direct employment of trial reported utilities and to represent the severity of the disease for patients and the health system. Patients in the sutimlimab arm started in the transfusion-dependent state and could move to the transfusion-independent state or stay in the transfusion-dependent state. We assumed that patients in the sutimlimab arm would never move from the transfusion-independent state back to the transfusion-dependent health state (i.e., 100% sustained sutimlimab efficacy). All patients in SOC arm started at the transfusion-dependent state and stayed there over their lifetime. Transition-state cycle was 1 month, chosen to reflect the 1–3 week time-period over which clinical effect of sutimlimab is and maintained thereafter.14, 15, 19 In each cycle, patients in both arms were subject to the risks of treatment- and transfusion-associated mortality, in addition to background mortality (Figure 1). We conducted our analysis over a lifetime time-horizon. TreeAge Pro Healthcare 2023 (TreeAge Software, Williamstown, MA) was used to build the model and conduct the analysis. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline was implemented where applicable.
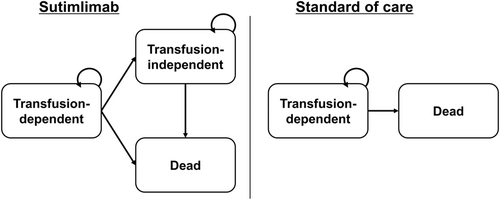
2.2 Model assumptions
In the sutimlimab arm, to favor the null hypothesis, we assumed that the efficacy of sutimlimab would persist over the patient's lifetime without drop-off (separately also considered over a 2.5-year time-horizon, informed by the pivotal trial and post-trial 2-year extension data, see Scenario Analyses) and that non-fatal adverse events due to sutimlimab treatment would not cause the discontinuation of sutimlimab treatment. Following the CARDINAL study protocol and FDA package insert, patients who weighed less than 75 kg at baseline received a 6.5 g dose of sutimlimab, while those who weighed 75 kg or more received 7.5 g.15 In our base-case analysis, we assumed a body weight of 64.8 kg to reflect the baseline characteristics of CARDINAL study participants (the higher body weight was tested in a separate scenario analysis, see Scenario Analyses).20 In the SOC arm, all patients undergo the costs and risks of chemoimmunotherapy with bendamustine-rituximab (rituximab 375 mg/m2 day 1 and bendamustine 90 mg/m2 days 1 and 2 for 4 cycles at a 28-day interval),12 followed by rituximab monotherapy (rituximab 375 mg/m2 days 1, 8, 15, and 22)21, 22 and subsequent treatment with mycophenolate mofetil (MMF) (500 mg twice daily),5 with all patients remaining dependent on transfusion care throughout (i.e., accruing those costs and risks as well). Severe adverse events due to bendamustine and rituximab, including infection, neutropenia,12 and progressive multifocal leukoencephalopathy (PML) were included.23 Furthermore, we conducted a separate scenario analysis where all patients in the SOC arm were pan-refractory to currently available treatment options,24, 25 sequenced to include bendamustine-rituximab, then bortezomib (1.3 mg/m,2 days 1, 4, 8, 11; discontinued after 3 months due to lack of efficacy),26, 27 then ibrutinib (420 mg daily; discontinued after another 3 months due to lack of efficacy),28 eculizumab (600 mg weekly 4 times, then 900 mg every other week; discontinued after another 3 months due to lack of efficacy),29 and MMF, all alongside transfusion care throughout. Oral acyclovir prophylaxis was administrated for patients on treatment with bortezomib and ibrutinib, as recommended.24
2.3 Clinical parameters
Base-case estimates and ranges for all input parameters used in our model are summarized in Table 1. The number of transfusions in both arms were informed by CARDINAL study data (mean 3.2 [range 1–19] for SOC, median 0 [range 0–13] for sutimlimab). Transfusion-dependent patients (i.e., all patients in SOC) could experience major transfusion-associated adverse events including delayed hemolytic transfusion reaction (DHTR), transfusion-associated circulatory overload (TACO), and transfusion-related acute lung injury (TRALI). Probabilities of incidence for these adverse events were obtained from hemovigilance reporting in the 2015 National Blood Collection and Utilization Survey.30 Mortality due to transfusion related adverse events were informed by published literature.31-34 Probability of PML incidence was obtained from a retrospective observational study which evaluated the incidence of PML in 976 patients with non-Hodgkin lymphoma treated with rituximab.23, 35 Given the uncertainty of the mortality due to PML in CAD patients, we assumed the worst case-fatality of 100% (i.e., to favor the null hypothesis), and evaluated this uncertainty in sensitivity analyses. We employed age- and sex-adjusted disease-specific background mortality based on the US Life Tables,36 along with a higher mortality risk with an adjusted hazard ratio of 2.35 (95% CI 1.34–4.13) for patients with primary CAD.37
| Base case values | Distribution used in probabilistic sensitivity analysis | References | |
|---|---|---|---|
| Clinical parameters | |||
| Patient age | 72 | Fixed | Roth 202115 |
| Patient body weight (kg) | 64.8 | Fixed | Sanofi20 |
| Body surface area (m2) | 1.7 | Fixed | Assumption |
| Transfusion free with sutimlimab | 0.708 | PERT (0.567, 0.850) | Roth 202115 |
| Transfusion free with SOC | 0 | Fixed | |
| Number of transfusions with SOC, per 6 months | 3.2 | Gamma (0.64, 5) | Roth 202115 |
| Number of transfusions with sutimlimab, per 6 months (for transfusion-dependent patients) | 0 | Gamma (0.13, 7.3) | Roth 202115 |
| Annual DHTR incidence, per product | 5.26E-05 | PERT (4.21E-05, 6.32E-05) | Goel 201930 |
| Annual TRALI incidence, per product | 1.75E-05 | PERT (1.40E-05, 2.11E-05) | |
| Annual TACO incidence, per product | 0.000111 | PERT (8.89E-05, 1.33E-04) | |
| DHTR mortality | 0.06 | PERT (0.048, 0.072) | Habibi 201631 |
| TRALI mortality | 0.075 | PERT (0.06, 0.09) | Popovsky 200832 |
| TACO mortality | 0.084 | PERT (0.067, 0.1) | 2021 SHOT Report33 |
| CAD mortality, HR | 2.35 | Fixed | Bylsma 201937 |
| Probability of PML incidence | 0.000199 | PERT (0.000159, 0.000239) | Focosi 2019, Tuccori 201023, 35 |
| PML mortality | 1 | PERT (0.7, 1) | Assumption |
| Utilities | |||
| Transfusion free state | 0.80447 | PERT (0.644, 0.965) | Roth 202249 |
| Transfusion dependent state | 0.7 | PERT (0.56, 0.84) | Roth 202249 |
| DHTR disutility | 0.4 | PERT (0.32, 0.48) | GBD 201952 |
| TRALI disutility | 0.4 | PERT (0.32, 0.48) | van Eerd 201051 |
| TACO disutility | 0.4 | PERT (0.32, 0.48) | |
| Chemotherapy disutility | 0.42 | PERT (0.16, 0.83) | Patel 202053 |
| PML disutility | 0.4 | PERT (0.3, 0.5) | Hamidi 201863 |
| Costs | |||
| Sutimlimab, per 10 mg ($) | 17.018 | Fixed | CMS39 |
| Sutimlimab dose, BW ≥75 kg (g) | 7.5 | Fixed | Roth 202115 |
| Sutimlimab dose, BW <75 kg (g) | 6.5 | Fixed | |
| Rituximab, per 10 mg ($) | 81.748 | Fixed | CMS39, 40 |
| Bendamustine, per 10 mg ($) | 18.274 | Fixed | |
| Bortezomib, per 0.1 mg ($) | 1.959 | Fixed | |
| Ibrutinib monthly cost ($) | 15 540 | Fixed | |
| Eculizumab, per mg ($) | 225.685 | Fixed | |
| MMF, per 250 mg ($) | 0.216 | Fixed | |
| Infusion cost, initial cost ($) | 64.72 | Gamma (100, 0.65) | CMS42 |
| Chemotherapy IV, first hour ($) | 142.55 | Fixed | |
| Chemotherapy IV, additional hour ($) | 30.68 | Fixed | |
| Chemotherapy IV, additional sequence ($) | 69.29 | Fixed | |
| RBC unit cost | 196.2 | Gamma (100, 1.96) | CMS41 |
| Transfusion administration cost | 1240 | Gamma (100, 12.4) | |
| Cost of DHTR | 1537 | Gamma (100, 15.4) | Janssen 201864 |
| Cost of TRALI | 9957 | Gamma (100, 99.6) | |
| Cost of TACO | 4830 | Gamma (100, 48.3) | |
| Adverse event due to bendamustine and rituximab therapy | 2758.26 | Gamma (100, 2.76) | CMS43 Berentsen 201712 |
| Monthly cost of healthcare resource utilization | 1694 | Gamma (100, 17) | Su 2020, Pham 20222, 44 |
| Cost of PML hospitalization ($) | 36 851 | Gamma (100, 369) | Chirikov 201947 |
| Mean hourly wage ($) | 29.76 | Fixed | U.S. Bureau of Labor Statistics48 |
| Cost of end-of-life care ($) | 50 678 | Gamma SD 12500 | Hogan 2001, Huntington 201845, 46 |
2.4 Costs
All costs were estimated in 2023 US dollars using the medical care component of the Consumer Price Index.38 Medication prices were obtained from the Centers for Medicare & Medicaid Services (CMS).39, 40 The cost of red blood cell products, blood transfusion administration, and infusion therapy were obtained from the 2023 CMS Hospital Outpatient Payment System41 and Physician Fee Schedule.42 The costs of severe adverse events due to bendamustine plus rituximab therapy were also incorporated into the model. We considered grade 3–4 neutropenia and infection, which is observed in the prospective multicenter clinical trial (CAD5 study),12 and then assumed that each adverse event (i.e., in the SOC arm) resulted in an inpatient hospitalization. Inpatient hospitalization costs associated with adverse events were informed by the 2022 CMS inpatient Prospective Payment System.43 Health resource utilization costs for SOC were further supplemented with country- and disease-specific real-world costs over a median of 42.8 and 33.0 months of follow-up after incident primary CAD diagnosis across two claims databases.2, 44 These SOC costs were specifically assessed from the highest of (1) annualized cost quartile and (2) the worst ordinal category of hemolytic anemia severity (defined as hemoglobin <8 g/dL) across all (1) hospitalizations, (2) emergency visits, (3) outpatient services, and (4) blood transfusions for patients with primary CAD. The cost of PML treatment and end-of-life care was based on prior literature.45-47 Lastly, we calculated the loss of productivity (i.e., caregiver) due to healthcare resource utilization and hospitalization by using mean hourly wage in the United States.48 We assumed that transfusion administration takes 4 hours and outpatient visits including chemotherapy administration and ER visits take 8 hours of caregiver working time, and varied these in sensitivity analyses.
2.5 Quality-adjusted life-years
Health outcomes were calculated in quality-adjusted life-years (QALYs), a measure that accounts for both health-related quality of life and length of life. Health-related quality of life was quantified by utility values, which range from 0 (death) to 1 (perfect health). EQ-5D-5L index scores obtained directly from the phase 3 CARDINAL trial were used to estimate the utilities of primary CAD patients in transfusion-dependent and transfusion-independent states, multiplicatively incorporating an age-dependent utility baseline also based on the EQ-5D-5L for the general population in the United States.49, 50 Disutilities due to transfusion related adverse events (i.e., in SOC) were informed by prior literature and accounted for the occurrence of all incident TRALI, TACO, and DHTR events.51, 52 We additionally incorporated a utility decrement of −0.42 during chemoimmunotherapy with bendamustine and rituximab, as previously employed for bendamustine-rituximab.53
2.6 Cost-effectiveness, threshold, sensitivity, and scenario analyses
We performed a cost-effectiveness analysis from the US healthcare sector perspective.54 Both cost and health outcomes were discounted by 3% annually.54, 55 We estimated the incremental cost-effectiveness ratio (ICER) in USD/QALY and determined cost-effectiveness using a willingness-to-pay threshold of $150 000/QALY. We performed one-way deterministic sensitivity analyses to examine the impact of parameter uncertainty on our results. All parameters were varied through at least a range of +/−20% of the base case estimates, and all parameters impacting the ICER at least 10% in either direction are reported. We additionally performed a 2-way sensitivity analysis ranging disease-specific utilities throughout their entire theoretical range for transfusion-free and transfusion-dependent states. This was followed with probabilistic sensitivity analysis to examine the effect of all uncertainty in the input parameters to the outcomes of our model. We defined appropriate probability distributions to all input parameters in our model, using beta-PERT distributions for transition probabilities and utilities, and gamma distributions for costs, varying all simultaneously across 10 000 Monte Carlo iterations. We then conducted two exploratory threshold analyses and four scenario analyses. The first threshold analysis solved for the (1) maximum sutimlimab cost threshold and (2) maximum sutimlimab treatment duration (i.e., after which all patients who achieved transfusion-free status with sutimlimab are assumed to maintain perfect remission without any sutimlimab). The second threshold analysis is informed by emerging data that some patients may be able to maintain hematologic remission after discontinuation of sutimlimab (reported out to 12 months).56 The four scenario analyses are (1) higher body weight patients weighing more than 75 kg (and receiving higher sutimlimab dosing of 7.5 g), (2) all-refractory SOC comprised of a treatment sequence of bendamustine-rituximab, followed by bortezomib, then ibrutinib, then eculizumab, and then MMF (all with ongoing transfusion support throughout), (3) incorporation of the caregivers' productivity loss (i.e., expanding to the US societal perspective), and (4) a 2.5-year time-horizon, aligning the duration of the pivotal trial and post-trial 2-year extension data.
3 RESULTS
3.1 Base-case and scenario analyses
In the base-case, treatment with sutimlimab versus SOC accrued 4.56 and 3.86 discounted QALYs at discounted costs of $1 980 000 and $336 000, respectively. The ICER for sutimlimab treatment in the base-case was $2 340 000/QALY [95% credible interval $1 070 000-12 400 000/QALY] (Table 2). In the scenario analysis for patients with higher body weight (i.e., higher sutimlimab dosing), the ICER was $2 770 000/QALY [95% CI $1 260 000-14 700 000/QALY]. In the scenario analyses for all refractory SOC through bendamustine-rituximab, bortezomib, ibrutinib, eculizumab, and MMF, discounted lifetime cost increased to $471 000, with an ICER for sutimlimab treatment of $2 150 000/QALY [95% CI $974 000–$11 400 000]. From the US societal perspective, discounted lifetime costs increased to $2 000 000 for treatment with sutimlimab, and $412 000 for SOC, respectively, with an ICER of $2 260 000/QALY [95% CI 1 020 000–12 000 000]. In the scenario analysis over a 2.5-year time horizon, consistent with the pivotal trial and extension study timeframe, treatment with sutimlimab versus SOC accrued 1.47 and 1.12 discounted QALYs at discounted costs of $618 000 and $143 000, respectively, yielding an ICER of $1 350 000/QALY [95% CI $754 000–$3 760 000].
| Strategy | Cost (USD) | Incremental cost (USD) | Effectiveness (QALYs) | Incremental effectiveness (QALYs) |
|---|---|---|---|---|
| Base-case analysis (patient body weight <75 kg) | ||||
| Standard-of-care | 336 000 | — | 3.86 | — |
| Sutimlimab | 1 980 000 | 1.65 million | 4.56 | 0.70 |
| Incremental cost-effectiveness ratio (ICER) = $2 340 000/QALY [95%CI 1 070 000–12 400 000] | ||||
| Scenario analysis (patient body weight ≥75 kg) | ||||
| Standard-of-care | 336 000 | — | 3.86 | |
| Sutimlimab | 2 280 000 | 1.94 million | 4.56 | 0.70 |
| Incremental cost-effectiveness ratio (ICER) = $2 770 000/QALY [95%CI 1 260 000–14 700 000] | ||||
| Scenario analysis (pan-refractory SOC) | ||||
| Standard-of-care | 471 000 | — | 3.86 | |
| Sutimlimab | 1 980 000 | 1.51 million | 4.56 | 0.70 |
| Incremental cost-effectiveness ratio (ICER) = $2 150 000/QALY [95%CI 974 000–11 400 000] | ||||
| Scenario analysis (societal perspective) | ||||
| Standard-of-care | 412 000 | — | 3.86 | |
| Sutimlimab | 2 000 000 | 1.59 million | 4.56 | 0.70 |
| Incremental cost-effectiveness ratio (ICER) = $2 260 000/QALY [95%CI 1 020 000–12 000 000] | ||||
| Scenario analysis (2.5-year time horizon) | ||||
| Standard-of-care | 143 000 | 1.12 | ||
| Sutimlimab | 618 000 | 475 000 | 1.47 | 0.35 |
| Incremental cost-effectiveness ratio (ICER) = $1 350 000/QALY [95%CI 754 000–3 760 000] | ||||
- Abbreviations: CI, credible interval; QALY, quality-adjusted life-year; USD, United States dollar.
3.2 Threshold and sensitivity analyses
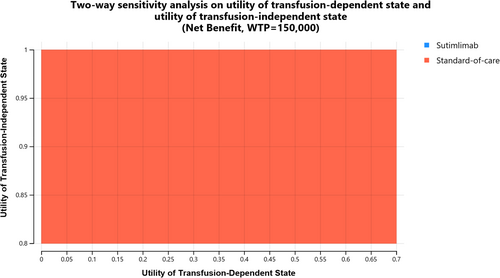
The results of one-way deterministic sensitivity analysis are shown in a tornado diagram (Supplemental Figure 1). We found that our model results are most sensitive to the utility of the transfusion-dependent and transfusion-independent state and the price of sutimlimab. In a probabilistic sensitivity analysis, SOC was favored over sutimlimab in 100% of 10 000 iterations (Supplemental Figure 2). These results also hold in all scenario analyses. Two-way sensitivity analysis on utility of the entire theoretical ranges of transfusion-dependent and transfusion-independent states shows no possible combination where sutimlimab is cost-effective (Figure 2). Threshold analyses showed that sutimlimab price needs to decrease by at least 80% or sutimlimab needs to be discontinued by 18 months of therapy and followed by lifelong treatment-free remission for sutimlimab to become cost-effective at the willingness-to-pay threshold of $150 000/QALY (Supplemental Figure 3).
4 DISCUSSION
This analysis represents, to the best of our knowledge, the first cost-effectiveness analysis of sutimlimab in CAD. We show that, by conventional cost-effectiveness standards, sutimlimab is never preferred over the standard-of-care for the treatment of transfusion-dependent CAD, with an ICER of $2 340 000/QALY over a lifetime time-horizon and $1 350 000/QALY over a 2.5-year time-horizon. These results are robust and consistent (i.e., no change in conclusion) across all deterministic and probabilistic sensitivity analyses, as well as all scenario analyses. Threshold analyses show that >80% price reduction or <18 months' treatment duration followed by lifelong treatment-free remission would be needed for this therapeutic to reach conventional cost-effectiveness, at a willingness-to-pay threshold of $150 000/QALY. The high drug cost of sutimlimab and the small increase in QALYs associated with sutimlimab use in the trial contribute to these findings, further reinforced by a two-way sensitivity analysis that allows for the entire incremental utility bound of up to 1. Despite demonstrating clinical benefit in phase 3 trials, sutimlimab remains a costly biologic agent, especially if administered over an indefinite period in CAD, calling for careful consideration of its value in the care of patients living with primary CAD.14, 15
These results should be viewed in light of the current landscape of CAD therapy, which includes chemoimmunotherapy and complement inhibition, as well as proteasome inhibitors and tyrosine kinase inhibitors, which are under investigation.57, 58 While sutimlimab is not cost-effective at its current pricing compared to the standard-of-care, that does not mean that it has no place in the treatment of patients with CAD. CAD is a heterogeneous disorder, and patients show considerable variability in response to recommended treatments, such as rituximab monotherapy.12, 57 Therefore, clinicians may choose a more personalized approach to CAD care, stratifying patients between B-cell-directed versus complement-directed therapies based on clinical phenotype. For example, patients experiencing hemolysis, thrombosis, or other symptoms related to chronic complement activation may be best managed with regimens that include classical complement pathway inhibition, such as sutimlimab. In comparison, other patients with more IgM-mediated symptoms, such as acrocyanosis, may be more suitable for B-cell or plasma cell-directed therapies.59 Sutimlimab may also find most value as a bridging agent to be used until B-cell-directed therapies take effect and so, pragmatically, as a tool for minimizing transfusion in patients presenting with severe anemia.57, 58
Evidence of disease recurrence following sutimlimab discontinuation in the CARDINAL 2-year extension study supports prior expectation that complement inhibition requires indefinite treatment for clinical effect maintenance (unless it is used as a bridge to B-cell or plasma-cell directed therapy).16, 58 Nevertheless, in our second threshold analysis solving for the maximum sutimlimab duration, we assume that 100% of patients can discontinue sutimlimab and maintain transfusion independence (off sutimlimab) for the rest of their lives, finding this threshold to be ~18 months. This assumption is structured to favor the null hypothesis and is based on an initial report of two patients total who displayed hematologic remission 12 months after treatment discontinuation.56 If this percentage is less than 100%, then the ICER rises and the cost-effective maximum treatment duration with sutimlimab decreases further. These findings further support our conclusion that on a population-level, sutimlimab is not cost-effective at current prices. For the subset of patients who may remain in remission following treatment cessation, the first clinical data has suggested the lack of IgM autoantibodies as one potential predictive marker in a small case series,56 theoretically supported by in vitro data of sutimlimab-mediated inhibition of primary B cell proliferation.60 Ultimately, pathophysiology-altering eradication of the pathogenic B-cell clone that allows sustained remission off lifelong therapy is the therapeutic goal for patients who require treatment for primary CAD. As with all therapeutic clinical decisions, these will continue to be balanced against the expected clinical risk–benefit associated with clone eradication, in the greater context of all available therapeutic possibilities. In the meantime, post-marketing real-world data surveillance of sutimlimab treatment in the CADENCE registry will hopefully accrue sufficiently long follow-up (planned for up to 6 years) to confirm both continued effectiveness (i.e., beyond 2 years in the extension studies) and to adjudicate any concerning safety signals of prolonged therapy which could have been missed in the time-limited phase 3 stage.59, 61 Any drop-off in effectiveness or cumulative toxicity with prolonged use would worsen the cost-ineffectiveness of sutimlimab, leading to larger health opportunity costs foregone by the greater at-risk population in the context of finite resources. In the light of new data, future clinical studies will allow updated economic evaluations to help inform both value- and/or equity-informed optimal treatment strategies, including that of various treatment sequences as the therapeutic armamentarium in primary CAD expands and longitudinal data accrues in the coming decades.58, 59
This study has several strengths. First, our analysis was based on the results of the latest phase 3 clinical trial evaluating the efficacy of sutimlimab in patients with a recent history of transfusion and that led to the FDA's approval of sutimlimab. We utilized baseline data and study-period data observed in the same patient population for both arms, which effectively abrogate concerns of confounding (short of secular trends in the same population within clinical trial duration) and so ensure the validity of the comparison between sutimlimab and SOC arms. Second, we incorporated utility values obtained directly from participants in the CARDINAL study. Third, we additionally quantified the loss of productivity. Given that primary CAD is a chronic condition in the elderly with significant potential impact on caregiver work participation, we believe the loss of productivity is an important factor for cost-effectiveness analysis for therapeutic interventions in primary CAD. Fourth, our results are universally consistent across extensive sensitivity and scenario analyses, inclusive of the short- and long-run time-horizons (i.e., 2.5-year and lifetime time-horizons) that are currently relevant in this field. We also recognize several limitations of our study, as well as areas for future research. First, our standard-of-care therapy sequencing of bendamustine-rituximab/rituximab monotherapy / MMF does not capture the entire spectrum of possible CAD treatment in transfusion-dependent patients, nor is it the sequence with which all patients are treated. However, we addressed this in our scenario analysis by examining a treatment sequence of bendamustine-rituximab, followed by bortezomib, then ibrutinib, then eculizumab and MMF, representing a pan-refractory course of therapy that yielded an ICER for sutimlimab that was lower, but still significantly above conventional thresholds. Further, our analysis focused on transfusion-dependent patients only who are, by definition, sicker at baseline than transfusion-independent patients, for whom it is reasonable to extrapolate that sutimlimab would be even less cost-effective compared to the SOC. In addition, by accounting for all CAD-specific health resource utilization costs and additionally accounting for treatment risk and cost (i.e., with SOC), we are very likely overestimating SOC cost: the ICER for sutimilimab versus SOC is likely greater than that reported here. Third, we assumed durable efficacy without fall-off in treatment response of sutimlimab over lifetime, given that the long-term effectiveness of sutimlimab is not conclusively established. However, if there is, in fact, fall-off in efficacy over time, this would only increase the ICER for sutimlimab treatment, which supports our conclusion that sutimlimab is not cost-effective at current pricing. With several limitations that actually strengthen our analysis and while our analysis demonstrated sutimlimab's cost-ineffectiveness from a conventional cost-effectiveness modeling standpoint, distributional cost-effectiveness analysis may potentially justify sutimlimab as an equitable therapeutic option in the context of a rare disease, as it has in the case of gene therapy for sickle cell disease in the context of a historically marginalized patient population.62
In summary, we performed the first cost-effectiveness analysis of sutimlimab versus standard-of-care in the transfusion-dependent CAD patient population. We found that at current pricing, sutimlimab is not cost-effective regardless of dosing (low and high-weight) and even when compared to pan-refractory SOC with intensive therapy and transfusion care, and over short- or long-run time-horizons. Significant price reduction (>80%) or time-limited treatment (<18 months) followed by lifelong treatment-free remission would be required to make sutimlimab a cost-effective treatment in the United States' patient population with primary CAD. An equity-informed analysis to derive an equity weight threshold for sutimlimab is an appropriate next step.
AUTHOR CONTRIBUTIONS
SI, DW, GG designed the study. SI, DW, AP, KC, AIL, AC, GG wrote and edited the manuscript.
ACKNOWLEDGMENTS
GG is supported by The Frederick A. DeLuca Foundation and The Yale Bunker Endowment.
CONFLICT OF INTEREST STATEMENT
AC has served as a consultant for MingSight, New York Blood Center, Pfizer, Sanofi, and Synergy and has received authorship royalties from UpToDate.
Open Research
DATA AVAILABILITY STATEMENT
For original data, please email the corresponding author.




