Suppression of Hb Bart's to improve tissue oxygenation and fetal development in homozygous alpha-thalassemia
Homozygous α-thalassemia is a lethal condition marked by severe intrauterine anemia and hypoxia, leading to fetal hydrops and mostly fetal demise. This is caused by a homozygous deletion in both α-globin genes, resulting in the production of Hb Bart's (γ4) instead of normal fetal hemoglobin (HbF, α2γ2).1 Hb Bart's has a high oxygen-affinity, inhibiting an effective tissue oxygen delivery.2 The introduction of intrauterine erythrocyte transfusions greatly improved survival and neurological outcome.3-5 Still, 5%–65% of survivors who received intrauterine transfusions are reported to have neurodevelopmental delay and growth restriction.3-5 We describe the pre- and postnatal management of homozygous α-thalassemia, targeted at suppressing fetal and neonatal hematopoiesis and reduce Hb Bart's percentage to <20%. Our treat-to-target approach was supported by real-time monitoring using Hb electrophoresis and reticulocyte counts. In contrast to regular Hb or hematocrit (Ht) based intrauterine transfusion regimens,6 our approach aimed at optimal tissue oxygen delivery, required for an improved fetal and postnatal development.2 This was followed by hematopoietic stem cell transplantation (HSCT) at the age of 6 months to cure the disease and prevent iron overload.
1 CASE REPORT
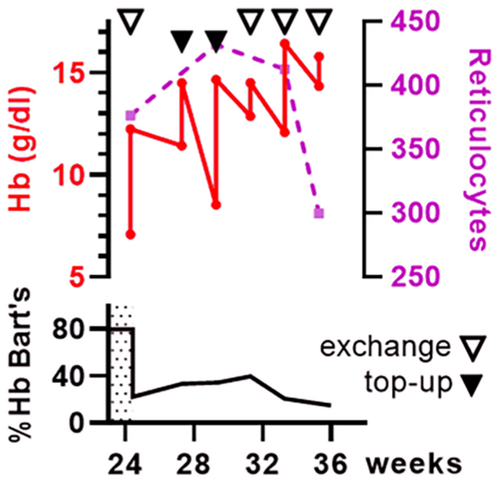
After 23 weeks of gestation, a non-consanguineous couple of Asian descent was referred to our tertiary care hospital carrying a male fetus diagnosed with homozygous α-thalassemia (Hb Bart's). Expert fetal ultrasound scans showed signs of chronic hypoxia (i.e., cardiomegaly, short long bones, mild periventricular whitening, and echogenic bowels). Peak systolic flow in the middle cerebral artery was normal. Whole exome sequencing revealed homozygous α-thalassemia. Parents were counseled and opted for fetal therapy. Confirmatory diagnostics using polymerase chain reaction and Hb electrophoresis after fetal umbilical cord blood sampling showed a homozygous ζ–SEA/ζ–SEA deletion and 80% Hb Bart's, respectively. The fetal Hb level was 7.1 g/dL with reticulocyte count of 376 × 109/L. At 24 weeks of gestational age (GA), the first intrauterine exchange transfusion (65 mL/kg estimated fetal weight) was performed and resulted in an increase of Hb to 12.3 g/dL with reduction of Hb Bart's to 22% (Figure 1). Fetal magnetic resonance imaging (MRI) at 26 weeks of GA showed no abnormalities except for fetal growth restriction and a gyration delay of 2 weeks of maturation. In line with international consensus,6 intrauterine top-up transfusions of 30 and 25 mL/kg fetal weight were administered at 27 and 29 weeks of GA, respectively. Despite top-up transfusions, persistent hyper-reticulocytosis >400 × 109/L and increasing pre-transfusion Hb Bart's levels to 40% revealed below-target suppression of the fetal hematopoiesis. Therefore, intrauterine exchange transfusions (50, 50, and 35 mL/kg fetal weight) were performed at 31, 33, and 35 weeks of GA, resulting in an gradual reduction of reticulocytosis and Hb Bart's level to 20% (Figure 1).
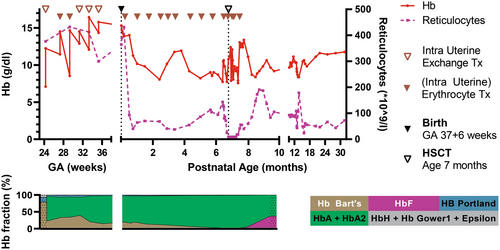
After 37 weeks and 6 days of pregnancy, labor was induced and a boy was born after an emergency caesarean section due to a suspected fetal stress. The neonate had a good start (APGAR 9/10), birth weight of 2490 gram (5th percentile (p5)), and Hb level 15.3 g/dL. Postnatal MRI showed normalization of the gyration pattern and no signs of hypoxia or iron overload. After birth, a 2- to 3-weekly erythrocyte transfusion scheme (10–15 mL/kg/transfusion) was initiated, resulting in an effective suppression of patient hematopoiesis and reduction of Hb Bart's to <10% (Figure 1). Simultaneously, donor search for HSCT was performed. To reduce toxicity of chemotherapy, the preparative regimen for HSCT was started when he reached 6 months of age and weighted 6 kg. Conditioning consisted of pre-conditioning with fludarabine 150 mg/m2 in 5 days and dexamethasone 125 mg/m2 in 5 days starting at day −30. Conditioning consisted of Thymoglobulin® 10 mg/m2 in 4 days from day −10, thiotepa 8 mg/kg on day −8, fludarabine 150 mg/m2 in 5 days from day −8, and treosulfan 30 mg/m2 in 3 days from day −7. At 7 months of age, a 11/12 HLA-matched cord blood unit of 2.9 × 106 CD34+ stem cells/kg was infused. Graft versus host disease prophylaxis consisted of ciclosporin A (target through level 150–200 μg/L) during 6 months and prednisolone (1 mg/kg/day) during 6 weeks.
Apart from a severe chemotherapy-induced intestinal mucositis, the early post-transplant course was uncomplicated. Hematologic and immunologic reconstitution was rapid, and the last erythrocyte transfusion was given 21 days after HSCT. In total, our patient received 20 blood transfusions and did not develop iron overload. His ferritin level was 826 μg/L at birth, was stable until transplantation, and spontaneously decreased to 171 μg/L at 2 years of age. In the months after HSCT, the patient developed food intolerance and oral food aversion, for which an ambulant multidisciplinary rehabilitation team was involved. Graft versus host disease was excluded in intestinal biopsies, and improvement was observed during a prolonged period of tube feeding.
Although follow-up is currently limited to 2.5 years, our patients length growth follows the p5 line of his reference curve, with normal weight-to length ratio and normal head circumference. At 2 years of age, Bayley Scales of Infant and Toddler Development (BSID III-NL) scoring was performed and showed cognitive development within normal range (score 96, 90% CI 89–104). Motor development is qualitatively normal, catching up a quantitative delay (score 78, 90% CI 73–87). He is included in an extensive follow-up program for monitoring the late effects of intensive treatment.
2 DISCUSSION
Here, we describe the successful management of an intrauterine diagnosed homozygous α-thalassemia with treat-to-target (exchange) transfusions, followed by HSCT in at infant age. Key to homozygous α-thalassemia is not only the anemia but also the high oxygen affinity of Hb Bart's leading to ineffective tissue oxygenation. Therefore, we aimed to suppress patient hematopoiesis and reduce Hb Bart's to <20% to maximize tissue oxygen delivery and support fetal- and postnatal development. Intrauterine exchange transfusions may carry an additional complication risk. Longer follow-up and larger patient numbers are required to confirm the beneficial effect of targeted intrauterine (exchange) transfusions as compared to regular top-up transfusions, especially in view of the potentially irreversible tissue damage resulting from chronic hypoxemia before the onset of intrauterine therapy. Although Hb electrophoresis may not always be readily available, hyper-reticulocytosis can be used as proxy for insufficient suppression of patient (Hb Bart's) hematopoiesis.2 In utero, our target was only reached with exchange transfusions. As expected, the transfused volumes were higher than current Hb/Ht-based intrauterine transfusion regimens.6 After birth, a hyper-transfusion regimen was required to reach our goal, which could be applied in the perspective of the upcoming HSCT to prevent chronic hyper-transfusion and iron overload. This targeted approach requires an intensive multidisciplinary collaboration of pediatric hematologists, fetal therapists, maternal–fetal medicine specialists, neonatologists, and laboratory specialists and may benefit the long-term outcome of upcoming therapies like in utero haploidentical HSCT as well.
FUNDING INFORMATION
No funding bodies were involved.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest apply.
PATIENT CONSENT STATEMENT
Parents consented with treatment and gave written informed consent for publication of this case report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.