An integrated therapeutic approach to sickle cell disease management beyond infancy
Abstract
Hydroxyurea, the first approved drug for sickle cell disease, decreases sickle hemoglobin polymerization by inducing fetal hemoglobin. Its effects in young children are excellent; responses in adults are variable and not curative. The goal of pharmacotherapy should not be disease “moderation” but reducing morbidity and mortality by diminishing both hemolytic anemia and vaso-occlusive events. This is best done by preventing sickle hemoglobin polymerization; if anti-polymerization treatment is insufficient, agents disrupting pathophysiologic pathways “downstream” of the sickle hemoglobin polymer should be added. We recommend that all patients should be started first on maximal doses of hydroxyurea. When the clinical and hematologic response to hydroxyurea is insufficient, as it is almost always in adults, we favor adding voxelotor, a hemoglobin-oxygen affinity-shifting agent that, likely in a pancellular distribution, decreases sickle hemoglobin polymerization. The P-selectin inhibitor crizanlizumab reduces sickle cell-endothelial interactions and can be used in patients with continued vaso-occlusive events. There is no physiologic reason that all three drugs could not be combined when the response to monotherapy or dual-drug therapy is poor. Drug therapy must be considered in the context of possibly “curative” cellular therapeutics and if needed, exchange transfusion programs.
1 INTRODUCTION
Linus Pauling, following a 1945 discussion with William Castle, first hypothesized that sickle cell disease (SCD) resulted from abnormal hemoglobin. Pauling recalled this conversation as “uninteresting,” but was sufficiently intrigued to begin laboratory studies that culminated in the identification of the first hemoglobin variant, sickle hemoglobin (HbS; α2βS2), and the initiation of “molecular medicine.”1, 2 Easy access to hemoglobin, globin mRNA and genomic DNA allowed SCD to be the first human disease characterized molecularly. The clinical use of ex vivo gene therapy in hematopoietic stem and progenitor cells (HSPC) appropriately extends the “firsts” attributed to SCD and other hemoglobin disorders. Forty-nine years elapsed from Pauling's discovery of HbS until hydroxyurea (HU), the first drug influencing the pathophysiology of the disease, was approved in the United States (US). Prior to approval, analgesics, transfusions, fluid replacement, penicillin prophylaxis, and antibiotics were the only widely available treatments.
Three recently FDA-approved drugs provide for the first time options for disease-modifying pharmacotherapy.3-5 In addition, progress in HSPC transplantation has improved its safety and efficacy increasing its use in children and adults with matched sibling donors.6 The promising results of gene therapy trials add another element for stakeholders to consider as this potentially “curative” therapeutic option moves toward FDA approval.7-12 We present a framework focusing on polypharmaceutical-based management of the “HbS-only” phenotype of SCD (HbS homozygosity and HbS-β0 thalassemia). It is difficult to create a “one size fits all” treatment. This framework might guide the use of combination disease-modifying therapies until curative options are safe, affordable, and widely available or until better drugs are developed. Two cases illustrate need for additional disease-modifying therapeutic approaches.
1.1 Case 1
A child homozygous for HbS was started on a maximally tolerated dose HU at 9 months of age. Fetal hemoglobin (HbF) was >30% and hemoglobin >9 g/dL. There were few disease manifestations until his mid-teens when he developed myelosuppression. A year later his hemoglobin was 6.2 g/dL, neutrophil count 1.3 × 109/L with increased reticulocytes and LDH. The HU dose did not increase because of concern for worsening neutropenia, and voxelotor was added with an increase in hemoglobin.
1.2 Case 2
A 34-year-old female with HbS-β0 thalassemia was first started on HU at age 30 after being referred to a sickle cell center. On 2000 mg HU/day, hemoglobin increased to 10.6 g/dL, absolute reticulocyte count was 200 × 109/L, and HbF was 24%. She continued to have 5 pain episodes annually requiring admission and asked about additional therapy. She was started on crizanlizumab infusions and over the course of 1 year she had 2 admissions for vaso-occlusive pain.
2 PATHOPHYSIOLOGY AND A PATHOPHYSIOLOGIC APPROACH TO TREATMENT
HbS polymerizes when deoxygenated with fascicles of polymer injuring the erythrocyte. HbS polymer leads both directly and indirectly to the multiple abnormalities of the sickle erythrocyte that contribute to the disease pathophysiology. Damaged sickle erythrocytes interact with other blood and endothelial cells, initiating the vascular pathophysiology of the disease, including acute painful vaso-occlusive episodes (VOEs) and acute chest syndrome (ACS). The survival of the sickle red blood cell (RBC) in the circulation is greatly reduced, resulting in chronic hemolysis, decreased nitric oxide bioavailability and release of immune system mediators that damage the endothelium and exacerbate vaso-occlusion and vasculopathy. Progressive vasculopathy is a significant contributor to chronic complications such as retinopathy, pulmonary hypertension and nephropathy, which often develop asymptomatically and may lead to organ failure. Preventing HbS polymerization decreases both VOEs and hemolytic anemia and is likely to be the most efficient form of treatment. Preventing effects “downstream” of polymerization, like intercellular adhesive interactions or the inflammatory response are useful but might be less efficacious than preventing the primary and indispensable pathophysiologic trigger.13 If HbS polymerization is prevented in nearly all RBCs there a few, if any, manifestations of SCD.7-11
2.1 Approved drugs
Four FDA-approved drugs are available (Table 1). HU induces high values of HbF (α2γ2) that decrease HbS polymerization, the primary driver of disease pathophysiology. Throughout the following discussion we emphasize that primary prevention of HbS polymerization, whether through induction of pancellular HbF expression, or through other means including increasing the O2 affinity of hemoglobin, currently provides the best chances of disease modification.
| Hydroxyurea | Voxelotor | Crizanlizumab | l-glutamine | |
|---|---|---|---|---|
| Route | Oral | Oral | Intravenous | Oral |
| Age of administration | 2-year approved age; 9 month practice guideline age | 4 year | 16 year | 5 year |
| Dosea | Daily, titrated to maximal subtoxic (≤35 mg/kg) | 1500 mg/day | 5 mg/kg/month | 5–15 g twice daily |
| Indication | All patients aged ≥9 months | See discussion | ||
| Mechanism of action | ↑ HbF | ↑ Hb-O2 affinity | P-selectin blockade | Unknown |
| ↓ HbS polymer | ↓ HbS polymer | ↓ Sickle cell adherence | ||
| Evidence of efficacy | ↓ VOE and hemolysis | ↓ Hemolysis | ↓ VOE | ↓ VOE |
| Cost/year ($)b | 1000 | 100 000 | 100 000 | 12 000–36 000 |
- a Consult prescribing information for dosing details for each drug.
- b Costs are approximate and highly likely to vary and change according to locale. VOE-vaso-occlusive events; HbS-Hemoglobin S; HbF Hemoglobin F.
2.1.1 Hydroxyurea
We might never have a better drug than HU, which is, cheap, and effective in “HbS-only” phenotypes and has been used safely for 30 years. HU produces stress erythropoiesis selecting erythroid progenitors that produce high values of HbF.14, 15 HbF has a primary effect on HbS polymerization. HU also provides disease control through reduced expression of surface molecules that lead to endothelial adhesion, decreased inflammatory response through suppression of leukocyte production, and increased vasodilatory compounds such as nitric oxide.15 Acute VOEs and hemolysis are decreased with reduced morbidity and mortality.15, 16 HU is the backbone of therapy and should be offered to all patients.17 Following its initial approval in selected middle age symptomatic adults in high-resource countries where it decreased VOE by 44%, significantly decreased the risk of severe ACS, the need for blood transfusions and hospitalizations, its use has spread throughout the world to all patients, symptomatic or not, and is now a standard of care.15, 18-24 HbF values achieved and the clinical and hematologic response to treatment vary according to age at initiation of therapy and dosing regimen (Table 2). Initiation of HU at 9 months of age has yielded outstanding results in patients followed up to 10 years. In a community-based study where HU was given to infants >9 months old who were followed for at least 6 years, HbF rose from 13 ± 8.7% to 26 ± 9.9% while hospitalizations decreased from 0.7 to 0.2 admissions per person-year, and the incidence of ACS decreased from 14.6 to 4.2 per 100 patients-years.24 By the last 2-year interval of follow-up, 72% of patients had total hemoglobin >9 g/dL, 42% had HbF >30%, 79% experienced no hospitalizations, and 94% received no transfusions.25 Despite its benefits HU is not a “cure.” Dosing in adults is often a challenge because of poor adherence, a dearth of knowledgeable adult providers, myelotoxicity, and diminished bone marrow reserve.16, 26-29 Male fertility has been a concern, however reduced sperm count is common in untreated SCD. A recent study evaluating semen volume and sperm count showed no significant difference in HU exposed and unexposed patients.30 Inconclusive data from small studies have suggested diminished ovarian reserve in female patients on HU; however how this translates to fertility outcomes remains unclear. We therefore do not routinely offer fertility preservation to our patients.31, 32
| Age treatment initiated | 9–26 months20, 21 | 12–17 years95, 96 | 18–49 years16, 97, 98 |
|---|---|---|---|
| % HbF likely to be achieved | 29.0→34.8 | 15.1, 17.3 | 5.1→8.7 |
| Hemoglobin (g/dL) | 9.2→10.1 | 9.4, 9.3 | 8.5→9.1 |
| Reticulocytes (109/L) | 275→157 | 8.7% | 327→231 |
| VOE reduction | 6.5/100 patient. years | 1.1→0.17 | Pain ↓44% |
| Events/person-year. during 10 years | ACS ↓50% |
- Note: Results are representative and illustrative of the change in selected parameters according to the age therapy was started. Some studies included a few patients with HbSC disease and HbS-β+ thalassemia. Other studies not cited here that are of different sizes, race/ethnicity, age distributions, drug dosing, adherence, laboratory procedures and definitions of clinical events could have somewhat different average values. VOE-vaso-occlusive events; ACS-acute chest syndrome; HbF Hemoglobin F.
2.1.2 l-glutamine
l-glutamine (Endari) was licensed in the US in 2017 for individuals 5 years of age or older with SCD but has not been approved in Europe.5 This agent might work by decreasing susceptibility of sickle erythrocytes to oxidative damage.33-36 In a pivotal double-blind phase III trial, oral l-glutamine administered twice daily reduced the incidence of VOEs and ACS and resulted in fewer hospitalizations and shorter duration of hospital stays compared to placebo over a 48-week period. The patients in the l-glutamine group had a median of 3 VOEs in the placebo group patients had a median of 4 VOEs. There were no significant between-group differences in the change in hemoglobin value, hematocrit, MCV, or reticulocyte count.5 Perhaps due to the twice-daily dosing regimen and powder formulation of the medication, adherence to treatment seems poor. Only 63.8% and 75.6% of patients in the l-glutamine and placebo arm respectively completed the Phase 3 trial. Fourteen months after patients were prescribed l-glutamine, 19% were actively on treatment, 42% discontinued, 35% never filled the prescription, and 4% had never started therapy.34, 37, 38
2.1.3 Voxelotor
Voxelotor (Oxbryta) was first approved in the United States in 2019 for adults and children 12 years of age or older and subsequently for children aged 4 years or older. Voxelotor binds the amino terminus of the α hemoglobin subunit, increasing the affinity of hemoglobin for O2. Increased hemoglobin-O2 affinity decreases deoxyHbS polymerization and red cell injury, and prolongs RBC half-life.39, 40 Patients of all ages who responded to treatment with voxelotor have shown increased hemoglobin values and reduced hemolysis.3, 40-43 In a phase 3, randomized, placebo-controlled, double-blind trial, 59% of patients aged 12–65 years treated with voxelotor had a hemoglobin increase greater than 1.0 g/dL at week 24 compared with 10.4% in the placebo group (p < .001).3, 41 Interestingly the long term follow-up results of this study, showed that the patients treated with Voxelotor were slightly more inclined to pursue treatment (53%) after 72 weeks than the patients treated with placebo (43%). Although patients continued reporting no significant change in pain events and quality of life, there was a sustained effect on hemoglobin values and hemolysis biomarkers. At 72 weeks 89% of those treated with voxelotor 1500 mg/day had a total hemoglobin increase of more than 1 g/dL.41 This might beneficially impact complications of hemolysis, like albuminuria.44 No significant reduction in VOCs was seen across treatment groups, but this phase 3 was not powered to demonstrate effects on VOCs. Whether voxelotor decreases the incidence of VOE is unclear.38, 40, 45 Patients treated with voxelotor 1500 mg with >12 g/dL hemoglobin had the lowest incidence of VOE.41, 46-48 Concerns about hypoxia and raised blood viscosity increasing acute VOEs remain hypothetical with little evidence that they will manifest. While we await more real-world data of voxelotor use in adults, we also await data from trials HOPE-Kids 1 and 2, which are ongoing and evaluate the use of voxelotor in children. Gastrointestinal side effects are most common; need for three pills daily, might impair drug adherence. Evaluation of voxelotor use in a real world setting demonstrated that 29 of 43 patients experienced at least one side effect, and in those with side effects 8 of 29 patients discontinued treatment.49
2.1.4 Crizanlizumab
Crizanlizumab (Adakveo) was approved in the US in 2019 in patients aged >16 years to reduce the frequency of acute VOE. This agent selectively binds P-selectin and blocks adherent interactions among endothelial cells, platelets, sickle erythrocytes and leukocytes.50 When given intravenously every 4 weeks there was a 45% reduction in the median annual rate of acute VOEs and a 42% reduction in the median annual rate of days hospitalized.4 The need for reliable venous access often limits the ability to deliver this drug especially in adults.51 Thirty-two percent of patients discontinued treatment for adverse events including infusion reaction and severe pain, or for perceived lack of benefit.52
2.2 Matched and haploidentical allogeneic HSPC transplantation
Allogeneic HSPC transplantation is the sole available “curative” treatment but requires careful risk/benefit assessment because of complications like graft-versus-host-disease (GVHD), graft failure, viral infection, secondary malignancies, and potential infertility.6, 53 Transplant- associated mortality is 5%–20% and usually due to complications of GVHD.54, 55 Patients with preexisting organ damage are most susceptible to these complications. In 1000 SCD patients who received allogeneic HSPC transplants from an HLA-matched sibling donor, 5-year probabilities of overall survival (OS) and event-free survival (EFS) were 92.9% and 91.4%, respectively. The 5-year OS was 95% and 81% for patients <16 years of age and those aged ≥16 years respectively; the corresponding EFS was 93% and 81%.56 A second study suggested that survival is best in patients under the age of 12 with a matched sibling donor.57 But, only approximately 14% of SCD patients have a matched sibling donor.58 Some centers recommend offering transplant to any symptomatic patient with a matched donor. This might be extended to asymptomatic patients without repetitive acute VOEs because of concerns about chronic disease progression. Others centers limit transplant to patients with “severe” disease, however this might be defined. Allogeneic transplant is usually indicated for pediatric and adult patients with an HLA-matched sibling donor with an overt stroke, frequent VOEs on HU and where organ damage has been documented or reduced lifespan is expected.6, 59 All SCD patients with full siblings should be assessed for possible allotransplant.
The use of alternative donors has been studied and should be reserved to a clinical trials settings for patients affected by a severe disease without an HLA-matched sibling donor. Matched unrelated donor transplant is associated with very high risk of acute and chronic graft versus host disease.60 Intra-familial, partially matched donors is associated with less favorable outcomes than those who have a fully matched sibling donor, although with increasing immunosuppression and post-transplant cyclophosphamide, haploidentical transplants are increasingly performed.61-65
2.3 Prophylactic transfusion
The goal of regular transfusions is to control the disease by diluting sickle RBCs. In comparison with manual blood exchange, erythrocytapheresis can decrease HbS% to <30% within a few hours and decrease the risk of iron overload in chronically transfused patients with sickle cell disease. Automated red blood cell exchange is not widely available because it often requires large central venous catheters, more packed RBC units and an apheresis device operated by specially trained staff.
Indications include neurological manifestations such as stroke and vasculopathy, recurrent severe VOEs despite maximum tolerated medical therapy, sometimes pregnancy, and perhaps uncontrolled disease on optimal medical therapy, such as progressive pulmonary hypertension, sickle nephropathy, or recurrent priapism. The potential for transfusion related complications, including delayed hemolytic transfusion reactions, alloantibody formation, iron overload, and chelation related toxicity, must be carefully monitored.66, 67 In patients on chronic transfusion considerations for a curative therapy should precede the development of severe iron overload and transfusion restrictions.
2.4 Gene therapies
The scarcity of fully matched donors and the requirement for post-transplant immunosuppression therapy leave room for improvement in HSPC transplantation in SCD. Although beyond our scope, in the near future for some patients different forms of gene therapy will be available, could be “curative” and will need to be considered in therapeutic planning.68, 69
Gene therapy uses autologous HSPCs that have been modified ex vivo with a viral vector or with DNA nucleases to obtain erythroblasts producing anti-sickling β-globin or γ-globin to dilute HbS and prevent its polymerization, giving rise to circulating RBCs with normal properties.
Lentiglobin (lovotibeglogene autotemcel) gene addition therapy, uses a lentiviral vector which encodes a modified beta globin gene, to produce anti-sickling hemoglobin HbAT87Q.7 The published results with Lentiglobin gene therapy showed that in the Group C of HGB-206 study, all patients followed for a median time of 17.3 months experienced engraftment and had hemoglobin increased from a median value of 8.5 g/dL to 11 g/dL or more 6–36 months after infusion. In 25 patients who were later evaluated, all described a resolution in VOEs.11
Gene editing, using CRISPR-Cas9 technology that targets fetal hemoglobin suppressor BCL11A showed HbF expression in a greater proportion of cells. Published interim results of the CLIMB study using CTX001 gene editing were showed promising results with over 99% pancellular HbF expression.9
The interim results of the study using gene silencing therapy were also recently published. Autologous CD34+ cells transduced with a BCH-BB694 lentiviral vector that encodes short hairpin RNA targeting BCL11A mRNA embedded in a microRNA, to allow for erythroid specific knockdown of BCL11A and upregulation of HbF. In six patients followed for a minimum of 6 months after therapy, all patients engrafted. All patients who were evaluated achieved an increased percentage of HbF in blood with values ranging from 20.4% to 41.3%.8
The conditioning regimens in published clinical trials used busulfan at myeloablative doses.
While gene therapy is not yet FDA approved for SCD, the patients should receive information about this potential future treatment. This would allow for time to prepare patients prior to myeloablative conditioning for gene therapy, insure those with iron overload are adequately chelated and optimize those with organ dysfunction. Gene therapy is not associated with GVHD as autologous cells are used. However, the risks of myeloablative chemotherapy, including secondary malignancy, organ damage, and infertility, remain a concern.
In the very first group of SCD patients treated in the US with ex vivo gene therapy, two patients with no significant positive effect of gene therapy, unfortunately developed myeloid neoplasms that were considered to be related to busulfan conditioning and not to the gene therapy, as they shared cytogenetic abnormalities characteristics of secondary myeloid leukemia.70, 71 Non-genotoxic and nonmyeloablative conditioning regimens remain an unmet need.68, 69
3 COMBINATION DRUG THERAPY
3.1 Criteria to add of voxelotor or crizanlizumab to HU
All patients should receive HU at maximally tolerated doses, regardless of age, or symptoms preferably starting at 6–9 months of age (FDA approval is for patients ≥2 years) Among infants who achieve total HbF values >30%, the clinical and hematologic response can be excellent. If these values of HbF are sustained most cells should have sufficient HbF to prevent HbS polymer-induced damage and it is unlikely additional drugs will be needed.72 However, there could be heterogeneity of clinical response even with these high HbF values.72 For example, following gene therapy that nearly totally reversed the phenotype of SCD, with total Hb F values around 40%, most sickle erythrocytes should have ≥10 pg HbF/F-cell, affording them nearly total protection from HbS polymerization. HU leads to greater heterogeneity of HbF values among F-cells than does gene therapy so that more cells can have HbF concentrations below those needed for full protection.15, 73 Many biomarkers are available to gauge the clinical responses to HU, but few, other than complete blood counts, reticulocyte count and HbF ≥30% are sufficiently reliable upon which to base therapeutic decisions.74, 75
Both the frequency of acute VOEs and the intensity of hemolysis should guide the addition of new agents to HU. More than a single VOE resulting in a healthcare provider encounter and ACS has been associated with increased risk of death, so prevention should be key.76 Hemolysis is associated with morbidity and mortality via well-studied pathophysiologic mechanisms.13, 77 Therefore, more than a “modest” amount of continued hemolytic anemia should provide an indication for additional treatment. Perhaps the reduction of hemolysis seen following gene therapy might serve as a benchmark until further observations can determine the risks, if any, of any mild residual hemolysis.7-11 Another benchmark might be to achieve hemoglobin values like those in most adults with HbSC disease where the prevalence of hemolysis-related complications is far less than in the “HbS-only” phenotypes.78-80 Benefits of reducing hemolysis on the development of its major associated complications like pulmonary vasculopathy and nephropathy are difficult to assess as this requires long-term follow-up.13, 77 (Figure 1).
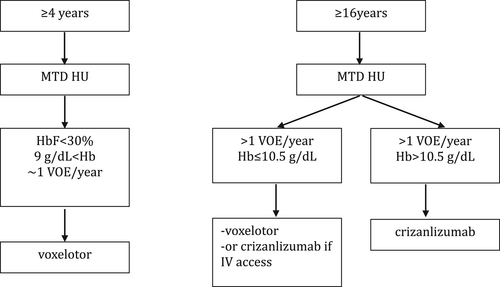
The outstanding results of HU monotherapy in infancy provide little imperative for pediatricians to add additional drugs. More often, waning benefits with aging often necessitate additional treatment.13, 81 We consider as indications for adding additional drugs more than one VOE per year requiring opioid infusion, hemoglobin concentration of <9 g/dL and HbF <30% of the total Hb. This reasoning is based on the association of VOEs and hemolysis with near and long-term morbidity and with mortality, and the association of high HbF values with milder disease. In contrast to HU monotherapy in children, in adolescence adherence to all medications including HU is rarely outstanding, and mostly insufficient. Despite hematologic and clinical improvement, VOE continue, hemolytic anemia persists, chronic complications happen, lifespan is shortened while costs of care increase continually.81-86 Health care providers face the dilemma of selecting which disease-modifying drug should be added to HU to remediate the shortcomings of monotherapy. In clinical trials, both voxelotor and crizanlizumab had additional therapeutic benefits when added to HU.3, 4 Combination drug regimens for SCD lack the guidance furnished by controlled clinical trials. A treatment paradigm, until “curative” cell-based therapeutics are widely available or new disease-modifying drugs are approved, is outlined in Figure 1.
Voxelotor alone among currently available options causes reductions in HbS polymerization that is likely to be pancellular.87-89 Based on the primacy of HbS polymerization in the pathophysiology of disease, when combination therapy is considered, we favor adding voxelotor first in patients aged <16 years. Voxelotor has been studied in patients with hemoglobin values <10.5 g/dL but should not be reserved for the most anemic patients. In the Phase 3 trial of voxelotor there was no increase in acute VOEs.
Patients, even those treated with HU at MTD, often seek additional treatment because of continued acute painful episodes but are less attuned to the impact of chronic hemolytic anemia and the long-term sequelae of intravascular hemolysis that are associated with mortality. Nevertheless, if VOEs continue and intravenous access is available crizanlizumab could be started. For patients with hemoglobin concentrations ≥10.5 g/dL, crizanlizumab might be used first. For patients aged ≥16 years with hemoglobin <10.5 g/dL, crizanlizumab could be discussed as an alternative to the addition of voxelotor. However, limited peripheral vein access and the need to have monthly treatment in an infusion center could be limiting.
The Phase 3 trial of voxelotor did not report on a reduction in acute VOE. Therefore, some favor crizanlizumab for patients with frequent pain while reserving voxelotor for patients who are most anemic. Continued follow up of voxelotor-treated patients suggested that those with the highest hemoglobin values had the most reduction in acute VOEs so this agent might be better than “neutral” regarding VOEs.41, 90 Some patients, for many reasons, will not take HU. They can be treated with voxelotor and crizanlizumab, alone or in combination.
3.2 Role of l-glutamine
Questions remain about the usefulness and mechanisms of action of l-glutamine.34 Its benefits are marginal and the potential for modifying the course of disease is unknown.5 If the clinical response to other combinations is poor, l-glutamine might be added to the treatment regimen of patients aged ≥5 years.
3.3 Triple therapy
Two-drug therapy is slowly becoming more common, but experience with three drugs is almost nonexistent. The use of three agents, each with a different mechanism of action and non-overlapping toxicities, is a paradigm that oncologists or other specialties have followed for decades and was suggested for SCD years ago.91, 92 It should be efficacious. Patients responding well to crizanlizumab will continue to have hemolysis and consideration should be given to adding voxelotor. Patients with reduced hemolysis during voxelotor treatment might continue to have an unacceptable rate of VOEs and benefit from crizanlizumab. The slow introduction of triple therapy in SCD, in contrast to the widespread use of multiple agents for hypertension, and cancer could be due to poor understanding of SCD pathophysiology and widespread misunderstanding among physicians of how best to confront the multipronged pathophysiology of this costly rare disease.92-94 Merely “moderating” vaso-occlusion and hemolysis is an unacceptable treatment endpoint. Adherence to any complicated treatment regimen is problematic. Taking many drugs is a burden. However, this is a severe and progressive disease. Better outcomes might incentivize adherence to treatment. Daily treatment burden might also make one-time curative treatments more acceptable.
3.4 Future development of combination drug therapy
One can reasonably assume that a clinical trial of triple therapy will never be done because of sponsor and investigator disinterest, and the limited time window for planning, enrollment, and completion of a Phase 3 clinical trial before “curative” treatments are approved and widely used. In lieu of a randomized controlled trial, it might be possible to assemble a registry of clinical, laboratory, and genetic information on SCD treated with HU, voxelotor, and crizanlizumab in different combinations by experienced clinicians at sickle cell centers and large medical centers. The effects of combinations of treatments on VOEs and hemolysis may provide useful information to guide treatment. If circumstances are right, they could be used to plan a multicenter clinical trial of the best treatment approaches. While we await further data on these drugs, other new drugs such as pyruvate kinase activators and HbF inducers are in clinical trials. If approved, they will provide additional opportunities for combination therapies.
4 CURATIVE TREATMENTS
While the above therapies can be tried, wherever possible curative therapy must be considered, especially in cases of young patients with emerging organ damage or significant SCD morbidity despite therapy. If the patient has an HLA-matched sibling, allogeneic stem cell transplantation can be performed. In experienced centers offering transplants to those with other donor sources, transplant can be considered. Otherwise, referral for gene therapy trials might be an option.
5 CONCLUSIONS
Based on the pathophysiology of SCD, combination drug therapy should be beneficial, however polypharmacy is not inexpensive. In the US there is a high rate of insurance denials of the newer agents due to perceived “insufficient medical necessity” or “medication not covered by the prescription plan.” The refusal of coverage is not pathophysiologically based and should be reversed by advocacy. Other barriers to proper treatment include too few hematologists, especially those treating adults, with deep understanding of this rare disease, a more general reluctance to push multiple therapeutics for a supposed “benign” disorder and patient inertia resulting from poor counseling about the disease's long-term and slowly developing consequences. Finally, difficulties with drug adherence are almost always seen with complex polypharmacy. The early results of cell-based treatments are promising but it is highly unlikely that when approved they will soon become effective treatments because of their cost and restriction to a very limited number of highly specialized centers. The same lentiviral vector-based gene therapy used in SCD clinical trials was FDA-approved for β thalassemia with an initial cost of $2.8 million/patient. Oral agents will be the core of management until DNA-based curative cell-based therapy is simplified, can be done in vivo, is proven safe, efficacious, long-lasting, widely available, and affordable. Until an oral HbF inducer that is at least as good as HU comes along, we will be using HU in some combinations with the currently available drugs or a similar group of agents that target hemoglobin-O2 affinity, cell adherence and perhaps inflammation.
AUTHOR CONTRIBUTIONS
Jean-Antoine Ribeil, Galia Pollock, Martin Steinberg, and Haydar Frangoul wrote and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
Jean-Antoine Ribeil: Bluebird Bio shareholder, consultant for Moderna Therapeuticx and AkiraBio. Galia Pollock: None. Martin Steinberg: consultant and advisory boards for Vertex Pharmaceuticals, Fulcrum Therapeutics and Astellas. Haydar Frangoul: consultant Vertex pharmaceuticals and Rocket Pharma, Speaker bureau Jazz Pharmaceutical.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




