A phase II study of sintilimab, anlotinib, and pegaspargase sandwiched with radiotherapy as first-line therapy in patients with newly diagnosed, stage I–II extranodal natural-killer/T-cell lymphoma
Peng Sun, Yajun Li, Cong Li, Kexing Ren contributed equally to this work.
Abstract
Novel highly effective and low-toxicity combination therapy for localized extranodal natural-killer/T-cell lymphoma (ENKTL) remains a clinically unmet need. This phase II trial (NCT03936452) investigated the efficacy and safety of sintilimab, anlotinib, and pegaspargase sandwiched with radiotherapy as first-line treatment in patients with newly-diagnosed stage I–II ENKTL. The patients received sintilimab 200 mg plus pegaspargase 2500 U/m2 on day 1 and anlotinib 12 mg once daily on days 1–14 for three 21-day cycles, followed by intensity-modulated radiotherapy and another three cycles of systemic therapy. The primary endpoint was the complete response rate (CRR) after six treatment cycles. The secondary endpoints included progression-free survival (PFS), overall survival (OS), CRR after two cycles, overall response rate (ORR) after six cycles, duration of response (DOR), and safety. Between May 2019 and July 2021, 58 patients were enrolled. The CRR was 55.1% (27/49) after two cycles and 87.8% (43/49) after six cycles. The ORR was 87.8% (43/49; 95% CI, 75.2–95.4) after six cycles. After a median follow-up of 22.5 months (95% CI, 20.4–24.6), the median PFS, OS, and DOR were not reached. The 2-year PFS, OS, and DOR rates were 87.6% (95% CI, 78.8–97.4), 97.9% (95% CI, 94.0–100), and 91.1% (95% CI, 83.2–99.8), respectively. Grade 3–4 treatment-related adverse events occurred in 41.4% (24/58) of patients, with the most common being hypertension (15.5%), hypertriglyceridemia (8.6%), oral mucositis (6.9%), and anemia (5.2%). No treatment-related deaths occurred. First-line sintilimab, anlotinib, and pegaspargase sandwiched with radiotherapy demonstrated promising efficacy in treatment-naïve early-stage ENKTL patients with a favorable safety profile.
Abbreviations
-
- AE
-
- adverse event
-
- CR
-
- complete response
-
- CRR
-
- complete response rate
-
- CT
-
- computed tomography
-
- CTCAE
-
- Common Terminology Criteria for Adverse Events
-
- CTV
-
- clinical target volume
-
- DOR
-
- duration of response
-
- EBV
-
- Epstein–Barr virus
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- ENKTL
-
- extranodal natural-killer/T-cell lymphoma
-
- FGFR
-
- fibroblast growth factor receptor
-
- GTV
-
- gross tumor volume
-
- HDACi
-
- histone deacetylase inhibitor
-
- IMRT
-
- intensity-modulated radiotherapy
-
- irAE
-
- immune-related adverse event
-
- ITT
-
- intent-to-treat
-
- LDH
-
- lactate dehydrogenase
-
- mITT
-
- modified intent-to-treat
-
- MRI
-
- magnetic resonance imaging
-
- NKTL
-
- natural killer/T-cell lymphoma
-
- NRI
-
- nomogram-revised risk index
-
- ORR
-
- overall response rate
-
- OS
-
- overall survival
-
- PD
-
- progressive disease
-
- PDGFR
-
- platelet-derived growth factor receptor
-
- PD-1
-
- programmed cell death-1
-
- PD-L1
-
- programmed cell death-ligand 1
-
- PET-CT
-
- positron emission tomography-computed tomography
-
- PFS
-
- progression-free survival
-
- PINK-E
-
- prognostic index for natural killer/T-cell lymphoma—Epstein–Barr virus
-
- PR
-
- partial response
-
- PTV
-
- planning target volume
-
- SAE
-
- serious adverse event
-
- SD
-
- stable disease
-
- TKI
-
- tyrosine kinase inhibitor
-
- TRAE
-
- treatment-related adverse event
-
- VEGFR
-
- vascular endothelial growth factor receptor
-
- 18FDG-PET
-
- 18F-fluorodeoxyglucose positron emission tomography
1 INTRODUCTION
Natural killer/T-cell lymphoma (NKTL) is a rare and distinct subtype of non-Hodgkin's lymphoma, and most NKTL cases are found in East Asia and Latin America.1, 2 In China, extranodal NKTL (ENKTL), a major subtype of mature T/NK cell lymphomas, is the second most common lymphoma after diffuse large B-cell lymphoma and accounts for approximately 11% of all lymphomas.3 Notably, 70%–90% of all ENKTL patients present with localized disease at the time of diagnosis.4, 5
Radiotherapy is the cornerstone for localized ENKTL.5, 6 However, approximately 40% of patients will experience disease recurrence or death within 5 years after radiotherapy alone.7 Asparaginase/pegaspargase-based chemotherapy is the standard of care for patients with treatment-naïve advanced ENKTL,8-11 and chemoradiotherapy has become a widely used treatment modality for early-stage ENKTL, with preferable clinical efficacy.7, 12 Nevertheless, the use of multiple cytotoxic agents will inevitably lead to high hematological toxicity and risk of infection, hindering the completion of the full-dose radiotherapy regimen, affecting the patient's adherence to the treatment protocol, and leading to a poorer prognosis. Improving the survival of patients with localized ENKTL remains an unmet clinical need and combining novel low-toxicity systemic therapy with radiotherapy may further improve the outcome of this patient population.
The Epstein–Barr virus (EBV) load is a significant prognostic factor for ENKTL.13 EBV can increase the expression of programmed cell death-ligand 1 (PD-L1) in several cancer types, including NKTL. In addition, PD-L1 overexpression is a potential mechanism of immune evasion.14-16 Some retrospective studies reported an overall response rate (ORR) of 57.1%–100% with anti-programmed cell death-1 (PD-1) antibody monotherapy in relapsed/refractory NKTL.17-20 An exploratory study showed an ORR of 88.9% with an anti-PD-1 antibody added to the P-GEMOX (pegaspargase, gemcitabine, and oxaliplatin) regimen in patients with advanced NKTL, and the 1-year progression-free survival (PFS) and overall survival (OS) rates were 66.7% and 100%,21 respectively. The ORIENT-4 study demonstrated that sintilimab (a recombinant humanized anti-PD-1 monoclonal antibody) monotherapy was effective and well-tolerated in patients with relapsed/refractory ENKTL, with an ORR of 75% and a 2-year OS rate of 78.6%.18
Antiangiogenic agents have not been commonly studied in NKTL, but the overexpression of angiogenesis-related genes has been observed in ENKTL.22 Anlotinib is a small-molecule tyrosine kinase inhibitor (TKI) that targets the vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), and platelet-derived growth factor receptor (PDGFR). The promising anti-tumor activity of anlotinib has been demonstrated in sarcoma and lung cancer.23, 24 Immune checkpoint inhibitors and antiangiogenic agents have shown synergistic effects in many tumor types,25-31 including the combination of sintilimab and anlotinib in treatment-naïve advanced non-small-cell lung cancer (ORR: 72.7%; median PFS: 15 months),26 treatment-naïve advanced hepatocellular carcinoma (ORR: 35.0%; median PFS: 12.2 months),29 recurrent or advanced endometrial cancer (ORR: 73.9%),30 and recurrent or metastatic cervical cancer (ORR: 54.8%; median PFS: 9.4 months).31 Therefore, we hypothesized that this synergistic effect might also be observed in ENKTL. Based on the above theories and encouraging clinical effects observed in other tumor types, we previously conducted a retrospective study that suggested that the combination of anti-PD-1 antibody, anlotinib, and pegaspargase sandwiched with radiotherapy was effective and well-tolerated in patients with localized ENKTL, with an ORR of 100%, a complete response rate (CRR) of 87.5%, and 3-year PFS and OS rates of 100%.32
Considering the promising preliminary results observed in our retrospective study, we conducted a prospective phase II trial to investigate the efficacy and safety of the anti-PD-1 antibody sintilimab, antiangiogenic agent anlotinib, and chemotherapeutic agent pegaspargase sandwiched with radiotherapy as first-line treatment in patients with newly diagnosed, early-stage ENKTL. Herein, the efficacy, survival, and safety results are reported.
2 METHODS
2.1 Study participants and design
This single-arm, open-label phase II trial enrolled patients at the Sun Yat-Sen University Cancer Center between May 2019 and July 2021. The eligible patients were (1) aged 18–70 years, (2) histologically confirmed ENKTL with clinical stage of I–II by computed tomography (CT) or magnetic resonance imaging (MRI) according to the Ann Arbor staging system, (3) Eastern Cooperative Oncology Group (ECOG) performance status 0–3, (4) estimated life expectancy >3 months, (5) at least one evaluable lesion according to the Lugano 2014 criteria (>15 mm or positive 18F-fluorodeoxyglucose positron emission tomography [18FDG-PET] uptake),33 (6) adequate organ and bone marrow functions, assessed within 14 days before starting the study treatment, (7) newly-diagnosed disease without previous anti-tumor therapy including radiotherapy, chemotherapy, targeted therapy, and stem cell transplantation, and (8) negative pregnancy tests (serum or urine) within 14 days before study enrollment for fertile women. Patients were excluded if they had (1) previous treatment with small-molecule TKIs, (2) a history of severe hemorrhage or any bleeding events of grade ≥3 according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 within 4 weeks before enrollment, (3) uncontrolled hypertension, (4) involvement of central nervous system, (5) active hemorrhage or at risk of fatal hemorrhage, (6) history of immunodeficiency, or (7) clinically significant cardiovascular diseases.
This study was approved by the ethics committee of Sun Yat-Sen University Cancer Center (approval number B2019-048-X01) and was registered at ClinicalTrials.gov (NCT03936452). It was conducted following the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines. All patients provided written informed consent prior to any study procedure. We have uploaded the essential raw data onto the Research Data Deposit (RDD2022191382) public platform (https://www.researchdata.org.cn).
2.2 Procedures
The eligible patients received sintilimab 200 mg intravenously on day 1, pegaspargase 2500 U/m2 intramuscularly on day 1, and anlotinib (12 mg once a day) orally on days 1–14 for three 21-day cycles, followed by intensity-modulated radiotherapy (IMRT) or 3D conformal radiotherapy (for patients who were unsuitable for IMRT). The planning target volume (PTV) was 54–56 Gy/25-26F for gross tumor volume (GTV), 50–50.7 Gy/25-26F for high-risk clinical target volume (CTV1), and 45 Gy/25-26F for low-risk clinical target volume (CTV2). GTV was determined based on nasal/nasopharyngeal and neck MRI combined with PET-CT and endoscopic findings. GTV included the primary tumor and involved regional lymph nodes. CTV1 included GTV and adjacent structures in risk, such as nasal mucosa, nasopharyngeal mucosa, retropharyngeal lymph nodes, the Waldeyer's ring, and ethmoid sinus. CTV2 included the corresponding neck lymphatic drainage area; the upper cervical lymph node was included when nasopharynx or retropharyngeal lymph node was involved and the whole cervical lymph node was included when an upper cervical lymph node was involved.34 Additional three cycles of systemic therapy with the same regimen were given after radiotherapy. Patients discontinued the study in case of disease progression, death, intolerable toxicity, or withdrew consent. Dose adjustments were allowed to manage adverse events (AEs), as detailed in the study protocol (Appendix 1).
2.3 Outcomes
The primary endpoint was CRR (defined as the percentage of patients with a complete response [CR]) after six cycles of treatment. The secondary endpoints included PFS (defined as the time from the first dose of study drug to disease progression or death from any cause, whichever occurred first. Patients who were event-free were censored at the time of last progression-free disease assessment), OS (defined as the time from the first dose of study drug to death. Patients who were event-free were censored at the last known date of being alive), CRR after two cycles of treatment, ORR (defined as the percentage of patients with a CR or partial response [PR]) after six cycles of treatment, duration of response (DOR, defined as the time from first documented CR or PR to disease progression or death), and safety.
Tumor response was assessed by PET-CT after every two cycles of treatment according to the Lugano 2014 criteria33 and classified as CR, PR, stable disease (SD), and progressive disease (PD). For patients with measurable lesions who had financial difficulties, CT was also acceptable considering the high cost of PET-CT. Tumor response and DOR were plotted by nomogram-revised risk index (NRI)35 or prognostic index for NKTL-EBV (PINK-E)36 (Appendix 2). Follow-up was performed every 3 months in the first 2 years, every 6 months during the next 3 years, and then annually. All AEs were assessed and graded according to CTCAE 5.0.
2.4 Statistical analysis
The historical CRR was estimated to be 70% because the threshold CRR was estimated to be 60%–80% based on the available CRR data for NKTL cases who underwent radiotherapy alone.7, 12, 37-45 Our trial was designed to have a statistical power of 90% to test the following two-sided hypotheses regarding the actual probability of an overall response; H0: p ≤ 70% (70% being the historical response while using conventional therapy) versus H1: P 85% (85% being the expected response), with a type I error of 0.1. The number of suitable patients required for our study was at least 49. Considering a 10% dropout rate, the required number of patients was 55.
The intent-to-treat (ITT) set included patients who received at least one dose of the study drug. The modified ITT (mITT) set was a subset of the ITT set, including patients who completed treatment and tumor assessment in accordance with protocol. The mITT set was used for evaluating the efficacy. Statistical analysis was performed using IBM SPSS 22.0, and graphical analysis was performed using R studio 4.2.0 software. Continuous variables were presented as median (range). Categorical variables were presented as n (%) and analyzed using the chi-square test or Fisher's exact test. The 95% confidence intervals (CIs) of CRR and ORR were calculated using the Clopper-Pearson method. A p < .05 was considered statistically significant. Kaplan–Meier method was applied to estimate PFS, OS, DOR, and their 95% CIs.
3 RESULTS
3.1 Patient characteristics
Sixty-one patients were screened; among them, 58 were included in the ITT set, 54 had at least one tumor assessment, and 49 were included in the mITT set (Figure S1). Of the ITT set (median age, 48.0 years [range, 20.0–70.0]), 36 (62.1%) were male, 38 (65.5%) had primary tumor invasion, 24 (41.4%) were Ann Arbor stage II, 27 (46.6%) had B symptoms, 22 (37.9%) had elevated lactate dehydrogenase (LDH), 27 (46.6%) had positive EBV, 12 (20.7%) had an NRI score of 3–4, and nine (15.5%) had a PINK-E score of 2 (Table 1).
| Characteristic | ITT (N = 58) | mITT (N = 49) |
|---|---|---|
| Age, years, median (range) | 48.0 (20.0–70.0) | 47.0 (20.0–69.0) |
| Male, n (%) | 36 (62.1) | 30 (61.2) |
| Primary tumor invasion, n (%) | ||
| Yes | 38 (65.5) | 33 (67.3) |
| No | 20 (34.5) | 16 (32.7) |
| Ann arbor stage at enrollment, n (%) | ||
| I | 34 (58.6) | 26 (53.1) |
| II | 24 (41.4) | 23 (46.9) |
| B symptoms, n (%) | ||
| Absent | 31 (53.4) | 26 (53.1) |
| Present | 27 (46.6) | 23 (46.9) |
| Serum LDH level, n (%) | ||
| Normal | 36 (62.1) | 30 (61.2) |
| Elevated | 22 (37.9) | 19 (38.8) |
| ECOG performance status, n (%) | ||
| 0 | 2 (3.4) | 2 (4.1) |
| 1 | 55 (94.8) | 46 (93.9) |
| 2 | 0 | 0 |
| 3 | 1 (1.7) | 1 (2.0) |
| Plasma EBV, n (%) | ||
| Positive | 27 (46.6) | 23 (46.9) |
| Negative | 30 (51.7) | 26 (53.1) |
| Missing | 1 (1.7) | 0 |
| NRI score, n (%) | ||
| 0–2 | 46 (79.3) | 39 (79.6) |
| 3–4 | 12 (20.7) | 10 (20.4) |
| PINK-E score, n (%) | ||
| Low risk (0–1) | 49 (84.5) | 42 (85.7) |
| Intermediate risk (2) | 9 (15.5) | 7 (14.3) |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; EBV, Epstein–Barr virus; ITT, intent-to-treat; LDH, lactate dehydrogenase; mITT, modified intent-to-treat; NRI, nomogram-revised risk index; PINK-E, prognostic index for natural killer/T-cell lymphoma-EBV.
3.2 Efficacy
All 54 response-evaluable patients completed radiotherapy. Fifty-two (96.3%) of 54 patients were evaluated by PET-CT, and 2 (3.7%) patients were evaluated by CT. After two cycles, the CRR and ORR were 55.1% (27/49) and 93.9% (46/49), respectively. The CRR increased to 81.6% (40/49) after four cycles, and the ORR was 87.8% (43/49). After six cycles, three patients with PR reached CR, with both CRR and ORR of 87.8% (43/49) (Table S1). For the five patients who were lost to follow-up after the first assessment, two achieved CR and three achieved PR.
No statistically significant was observed with respect to subgroup analyses of CRR after six treatment cycles. CRR was numerically higher in patients with stage I than in those with stage II disease (92.3% vs. 82.6%, p = .400). Patients with elevated LDH had numerically higher CRR than those with normal LDH (94.7% vs. 83.3%, p = .384). Patients with positive EBV had numerically higher CRR than those with negative EBV (91.3% vs. 84.6%, p = .671). The CRR was numerically higher in patients with an NRI score of 0–2 than in those with an NRI score of 3–4 (89.7% vs. 80.0%, p = .588). The ORR showed similar results (Table S2).
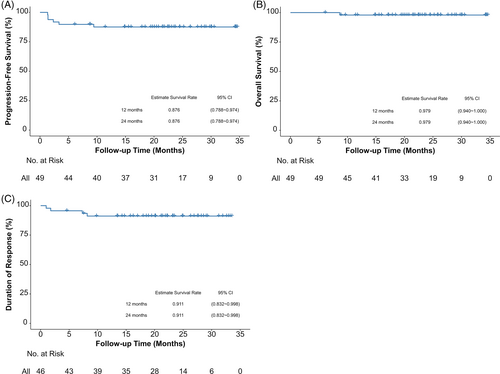
After a median follow-up of 22.5 months (95% CI, 20.4–24.6), the median PFS and OS were not reached. The 2-year PFS and OS rates were 87.6% (95% CI, 78.8–97.4), and 97.9% (95% CI, 94.0–100), respectively. The median DOR was not reached and 2-year DOR rate was 91.1% (95% CI, 83.2–99.8) (Figure 1). For the patients who achieved CR after six cycles of treatment, 39/43 (90.7%) remained disease-free and received follow-up. The swimmer plots for tumor response and DOR by NRI score or PINK-E score are shown in Figure 2.

3.3 Safety
Among the ITT set, any grade treatment-related AEs (TRAEs) were reported in 86.2%, with the most common being oral mucositis (n = 28 [48.3%]), hypoalbuminemia (n = 27 [46.6%]), alanine aminotransferase increased (n = 23 [39.7%]), nausea (n = 22 [37.9%]), hypertension (n = 21 [36.2%]), anorexia (n = 21 [36.2%]), abnormal thyroid function (n = 21 [36.2%]), hypertriglyceridemia (n = 21 [36.2%]), rash (20 [34.5%]), and anemia (n = 20 [34.5%]).
Grade 3–4 TRAEs were reported in 24 (41.4%) patients. The most common grade 3–4 TRAEs were hypertension (n = 9 [15.5%]), hypertriglyceridemia (n = 5 [8.6%]), oral mucositis (n = 4 [6.9%]), and anemia (n = 3 [5.2%]) (Table 2). Serious AEs (SAEs) occurred in four (6.9%) patients. No treatment-related deaths occurred.
| Event | Toxicity incidence, n (%) | |
|---|---|---|
| All grades (N = 58) | Grade 3–4 (N = 58) | |
| Any event | 50 (86.2) | 24 (41.4) |
| Hematological | 28 (48.3) | 4 (6.9) |
| Anemia | 20 (34.5) | 3 (5.2) |
| Neutropenia | 15 (25.9) | 1 (1.7) |
| Thrombocytopenia | 6 (10.3) | 1 (1.7) |
| Non-hematological | 50 (86.2) | 21 (36.2) |
| Oral mucositis | 28 (48.3) | 4 (6.9) |
| Hypoalbuminemia | 27 (46.6) | 1 (1.7) |
| Alanine aminotransferase increased | 23 (39.7) | 0 |
| Nausea | 22 (37.9) | 0 |
| Hypertension | 21 (36.2) | 9 (15.5) |
| Anorexia | 21 (36.2) | 1 (1.7) |
| Hypoparathyroidism | 21 (36.2) | 0 |
| Hypertriglyceridemia | 21 (36.2) | 5 (8.6) |
| Rash | 20 (34.5) | 1 (1.7) |
| Emesis | 17 (29.3) | 0 |
| Hemorrhage | 16 (27.6) | 0 |
| Direct bilirubin increase | 15 (25.9) | 0 |
| Fatigue | 15 (25.9) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 13 (22.4) | 2 (3.4) |
| Total bilirubin increase | 10 (17.2) | 0 |
| Diarrhea | 10 (17.2) | 0 |
| Fibrinogen decrease | 8 (13.8) | 0 |
| Infusion-related fever | 8 (13.8) | 0 |
| Lipase increase | 5 (8.6) | 0 |
| Proteinuria | 4 (6.9) | 0 |
| Thromboembolic event | 3 (5.2) | 0 |
| Serum amylase increase | 2 (3.4) | 0 |
| Oral pain | 2 (3.4) | 0 |
| Cerebral infraction | 2 (3.4) | 2 (3.4) |
| Acute pancreatitis | 1 (1.7) | 1 (1.7) |
| Shingles | 1 (1.7) | 1 (1.7) |
Immune-related AEs (irAEs) were all grade 1 or 2, including abnormal thyroid function (n = 21 [36.2%]), rash (n = 11 [19.0%]), and infusion-related fever (n = 8 [13.8%]).
A low incidence of hematological toxicities (48.3%) was observed in this study, including anemia (n = 20 [34.5%]), neutropenia (n = 15 [25.9%]), and thrombocytopenia (n = 6 [10.3%]). Only four (6.9%) patients suffered grade 3–4 hematological toxicities, with the most common being anemia (n = 3 [5.2%]).
Owing to AEs, 27 (46.6%) patients had dose delays, nine (15.5%) had study drug dose reduction, and six (10.3%) discontinued the study treatment.
4 DISCUSSION
To the best of our knowledge, this was the first study to investigate the efficacy and safety of a sintilimab-based regimen in the first-line treatment of early-stage ENKTL. The results confirmed the findings of our previous retrospective study32 that suggested a highly active triplet regimen (sintilimab, anlotinib, and pegaspargase) for treatment-naïve patients with early-stage ENKTL. The current results showed that the CRR and ORR were both 87.8% after six treatment cycles, with a 2-year PFS rate of 87.6% and a 2-year OS rate of 97.9%. Regarding safety, our study treatment resulted in a low rate of grade 3–4 hematological toxicities (6.9%), and all patients received full-dose and full-course radiotherapy at the protocol-specified time points. It might be attributed to the mild myelosuppression of the treatment regimen. Furthermore, the addition of combined radiotherapy led to the increase of CRR from 55.1% after two cycles of systemic therapy to 81.6%, which was close to 87.8% after the full six cycles of treatment. All these results suggested a feasible and promising treatment strategy for treatment-naïve localized ENKTL.
L-asparaginase/pegaspargase is the backbone component for ENKTL, and L-asparaginase/pegaspargase-based regimen has been recommended as standard treatment in patients with advanced ENKTL.5, 46 Compared with L-asparaginase, pegaspargase is more convenient for administration and has a relatively lower risk of allergy and is, therefore, mainly used at our institution. Although the L-asparaginase-based SMILE regimen (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) is effective for ENKTL,8, 9 the hematological toxicities are high (grade 3–4 neutropenia: 67%–100%; grade 3–4 thrombocytopenia: 42%–64%). A randomized controlled trial confirmed that the pegaspargase-based DDGP regimen (dexamethasone, cisplatin, gemcitabine, and pegaspargase) showed significantly better survival benefit (PFS: not reached vs. 6.8 months; OS: not reached vs. 75.2 months), higher ORR (90.0% vs. 60.0%), and lower hematological toxicities (leukopenia, 62.5% vs. 85.0%; neutropenia, 65.0% vs. 85.0%) compared with the L-asparaginase-based SMILE regimen in patients with newly-diagnosed advanced ENKTL,47 supporting the use of pegaspargase as primary chemotherapy in our study. Compared with these chemotherapy regimens with or without radiotherapy, our regimen with pegaspargase as a single chemotherapeutic drug sandwiched with radiotherapy could result in lower toxicity, leading to better compliance with the treatment protocol and sustained efficacy (2-year PFS rate of 87.6%, 2-year OS rate of 97.9% and 2-year DOR rate of 91.1%).
Immunotherapy has revolutionized cancer treatment, and anti-PD-1 antibodies have become effective salvage treatments in relapsed/refractory ENKTL. Using an anti-PD-1 antibody can reverse the inhibition of T-cell viability in EBV-positive diffuse large B-cell lymphoma.48, 49 In ORIENT-4, sintilimab monotherapy was reported to achieve an ORR of 75% and a 2-year OS rate of 78.6% in relapsed/refractory ENKTL.18 In an exploratory study of patients with advanced NKTL, sintilimab plus the P-GEMOX regimen resulted in an ORR of 88.9% and a 1-year OS rate of 100%.21 The SCENT study demonstrated effective anti-tumor activity and manageable safety profile of sintilimab plus chidamide (a histone deacetylase inhibitor [HDACi]) in the treatment of relapsed/refractory ENKTL, with an ORR of 58.3% and estimated 1-year OS rate of 79.1%.50 The subsequent SCENT-2 study investigated induction therapy with sintilimab plus chidamide followed by the P-GEMOX regimen in patients with newly diagnosed ENKTL. For early-stage disease, the ORR was 73.7% after two cycles of sintilimab plus chidamide and 100% after the whole treatment (sintilimab plus chidamide followed by P-GEMOX regimen involved field radiotherapy). For advanced disease, the ORR was 44.4% after three cycles of sintilimab plus chidamide and 100% with sintilimab plus chidamide followed by the P-GEMOX regimen. The estimated 1-year OS rate was 92.9% in the whole population.51 Hence, the evidence supports the use of sintilimab as a component of first-line treatment for patients with ENKTL.
Anlotinib, an antiangiogenic agent, can downregulate the expression of PD-L1 through the inactivation of the AKT pathway and remodel the tumor microenvironment.52 It can also increase the infiltration of innate immune cells to enhance the effect of PD-1 blockade.53 The synergistic effect of sintilimab and anlotinib has been reported in many cancers. First-line sintilimab plus anlotinib resulted in an ORR of 72.7% and a median PFS of 15 months in advanced non-small-cell lung cancer,26 and 35.0% and 12.2 months in advanced hepatocellular carcinoma.29 An ORR of 73.9% was observed with this combination in patients with recurrent or advanced endometrial cancer who progressed after platinum-based chemotherapy, and the median PFS was not reached with a median follow-up of 15.4 months.30 In recurrent or metastatic cervical cancer, second or later-line sintilimab plus anlotinib showed an ORR of 54.8% and a median PFS of 9.4 months.31 Our study also supported this synergistic effect, a rapid response was observed in our study after two cycles of sintilimab plus anlotinib and pegaspargase, with a higher short-term ORR (93.9% vs. 73.7%) compared with two cycles of chemotherapy-free regimen (sintilimab plus chidamide) in the SCENT-2 study.51
Local radiotherapy can induce an abscopal effect, leading to the regression of non-irradiated distant lesions. This abscopal effect is considered a systemic immune response. Although the exact mechanism of the abscopal effect remains unclear, the combination of immune checkpoint inhibitors and radiotherapy can boost the abscopal effect, extending the use of radiotherapy to treat both local and metastatic lesions.54, 55 Taken together, the results showed that our study treatment comprising of anti-PD-1 antibody, antiangiogenic agent, and pegaspargase sandwiched with radiotherapy is a promising treatment for treatment-naïve early-stage ENKTL.
The timing of local radiotherapy combined with systemic therapy is not standardized. Kwong et al. found that concurrent chemoradiotherapy could achieve better PFS and CRR than sequential modalities (chemotherapy followed by radiotherapy or radiotherapy followed by chemotherapy) in patients with early-stage ENKTL.56 On the other hand, patients who underwent concurrent chemoradiotherapy had a lower tumor burden (more patients with EBV-negative disease and low PINK-E score).56 Multivariable and Cox regression analyses showed that different treatment modalities resulted in comparable PFS and CRR.56 A meta-analysis compared sequential chemoradiotherapy with sandwiched chemoradiotherapy in patients with stage IE-IIE ENKTL and showed comparable CRR and 3-year PFS and OS rates between the two modalities.57 However, patients with sandwiched chemoradiotherapy had less hematological toxicity (15.3% vs. 27.9%).57 In addition, more patients with sandwiched chemoradiotherapy had Ann Arbor stage IIE disease, an independent factor associated with PFS.57 If the toxicity of a sandwiched modality is comparable to or better than sequential or concurrent modality, we assumed that the sandwiched modality would result in a better efficacy.
In this study, sintilimab, anlotinib, and pegaspargase sandwiched with radiotherapy showed a lower incidence of grade 3–4 AEs compared with the P-GEMOX, DDGP, and SMILE regimens, especially for grade 3–4 hematological toxicities (anemia: 5.2% vs. 17.1%–52.3%; neutropenia: 1.7% vs. 29.9%–100%; thrombocytopenia: 1.7% vs. 17.9%–63.2%).8-11, 21, 47 Consistent with previous studies of sintilimab plus anlotinib,26, 29-31 the irAEs in our study were all mild. In terms of anti-angiogenic TRAEs, the incidences of hypertension (36.2% vs. 19.0%–39.1%) and hemorrhage (27.6% vs. 26.1%–59.1%) were comparable to previous reports.26, 29-31 Thromboembolic events were also infrequent in our study, and the rate of proteinuria (6.9% vs. 18.2%–55.0%) was relatively lower than in previous reports.26, 29-31 No new safety signal was observed in this study. All these results suggested that the study regimen was well-tolerated.
This study had some limitations. It was a single-arm study without direct control groups for comparison. The sample size was relatively small, and the follow-up duration was short. The treatment of early-stage ENKTL in clinical practice might be different between China and Western countries (such as United States), thus the results in our study should be interpreted and concluded with cautions. Further studies in different populations and large-scale randomized controlled trials are warranted to validate our results.
In summary, first-line sintilimab, anlotinib, and pegaspargase sandwiched with radiotherapy are well-tolerated, and patients with treatment-naïve, stage I–II ENKTL can achieve a high CRR and persistent survival benefit. The synergistic effect of an anti-PD-1 antibody combined with an antiangiogenic agent is promising in this population and does not increase toxicity risk when added to chemoradiotherapy.
AUTHOR CONTRIBUTIONS
Zhi-Ming Li, Hui Zhou, Haiyan Yang and Liqun Zou had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Peng Sun, Yajun Li, Cong Li and Kexing Ren were involved in the study concepts and design. Peng Sun, Yu Wang, Wenqi Jiang and Haiyan Yang performed the statistical analysis and drafted the manuscript. All authors read, critically revised and approved the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Han-Yu Wang for his help in radiotherapy and all the patients and their families for participating in this study.
FUNDING INFORMATION
This work was supported by grants from National Science and Technology Major Project (nos. 2018ZX09734003), the National Natural Science Foundation of China (nos. 81872902, 82073917, 82103579, 82104273).
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
PATIENT CONSENT STATEMENT
All patients provided written informed consent prior to any study procedure.
CLINICAL TRIAL REGISTRATION
The study was registered at ClinicalTrials.gov (NCT03936452).
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and supplementary materials.




