Clinical outcomes after 4.5 years of eliglustat therapy for Gaucher disease type 1: Phase 3 ENGAGE trial final results
Clinical trial registration: NCT00891202
Abstract
Eliglustat, an oral substrate reduction therapy, is approved for eligible adults with Gaucher disease type 1. In the Phase 3 ENGAGE trial of previously untreated adults with Gaucher disease type 1, eliglustat-treated patients had statistically significant improvements in organ volumes and hematologic parameters compared with placebo in the 9-month primary analysis. We report final outcomes by time on eliglustat among all patients who participated in the ENGAGE trial and extension. No patient deteriorated clinically or withdrew due to adverse events; 39/40 patients entered the open-label extension period and 34/40 (85%) remained in the trial until completion or switching to commercial eliglustat after its approval (2.3–6 years). Clinically meaningful improvements in Gaucher disease manifestations were seen in all patients concomitant with reductions in pathological lipid substrate levels (glucosylceramide and glucosylsphingosine). Among patients with 4.5 years of eliglustat exposure, mean spleen volume decreased by 66% (from 17.1 to 5.8 multiples of normal [MN], n = 13), mean liver volume decreased by 23% (from 1.5 to 1.1 MN, n = 13), mean hemoglobin increased 1.4 g/dl (from 11.9 to 13.4 g/dl, n = 12), mean platelet count increased by 87% (from 67.6 to 122.6 × 109/L, n = 12), median chitotriosidase decreased by 82% (from 13 394 to 2312 nmol/h/ml, n = 11), median glucosylceramide decreased by 79% (from 11.5 to 2.4 μg/ml, n = 11), median glucosylsphingosine decreased by 84% (from 518.5 to 72.1 ng/ml, n = 10), and mean spine T-score increased from −1.07 (osteopenia) to −0.53 (normal) (n = 9). The magnitude of improvement in Gaucher disease manifestations and biomarkers over time was similar among the full trial cohort. Eliglustat was well-tolerated and led to clinically significant improvements in previously untreated patients with Gaucher disease type 1 during 4.5 years of treatment.
Video Abstract
Clinical outcomes after 4.5 years of eliglustat therapy for Gaucher disease type 1: Phase 3 ENGAGE trial final results
by Mistry et al.1 INTRODUCTION
In Gaucher disease, biallelic mutations in the GBA1 gene lead to defective acid β-glucosidase and lysosomal accumulation of glucosylceramide and glucosylsphingosine with immediate and downstream metabolic consequences.1 This metabolic defect underlies a multisystemic phenotype involving myeloid cells, most conspicuously seen as the lipid-engorged macrophages (i.e., Gaucher cells) and other cells, including neurons and mesenchymal-derived cells.2 The accumulating glycosphingolipids trigger immune activation involving myeloid cells, lymphocytes and possible complement activation that causes increased activity of glucosylceramide synthase, an effect that further amplifies the metabolic defect.3 Life-long intravenous infusions of macrophage-targeted enzyme replacement therapy (ERT) ameliorate hepatosplenomegaly, thrombocytopenia, anemia, some aspects of bone disease, and growth failure (in children) in type 1, non-neuronopathic Gaucher disease.4-7
Eliglustat is a specific inhibitor of glucosylceramide synthase and an oral first-line treatment for adults with Gaucher disease type 1 who have an extensive, intermediate or poor CYP2D6 metabolizer phenotype (>90% of patients8, 9).10-12 First approved in the United States in 2014 and the European Union in 2015, eliglustat is currently approved in more than 55 countries. Eliglustat offers a daily oral treatment alternative to one to two monthly infusions of ERT with macrophage-targeted recombinant human acid β-glucosidase, which has been the mainstay of Gaucher disease treatment for nearly three decades. An extensive clinical trial program of four Phase 2 and Phase 3 eliglustat trials involving 393 patients demonstrated the efficacy of eliglustat to ameliorate hematologic, visceral, and bone manifestations of Gaucher disease in treatment-naïve patients, as well as maintain the stability of these parameters in patients who switched from ERT to eliglustat.13-21 These include the ENGAGE trial, the only placebo-controlled trial ever conducted in Gaucher disease patients. In the 9-month, double-blind, primary analysis, eliglustat treatment in treatment-naive adults with Gaucher disease type 1 compared with placebo resulted in statistically significant improvements in spleen volume, liver volume, hemoglobin concentration, and platelet count, with commensurate reductions of lipid substrate levels and inflammatory biomarkers.15 During the 18-month open-label period, former placebo patients who switched to eliglustat showed similar time course and magnitude of improvement as the original eliglustat patients in the primary analysis, while patients initially randomized to eliglustat continued to improve during an additional 9 months of treatment.20
We report the final safety and efficacy outcomes for patients in the ENGAGE trial by cumulative time on eliglustat, which ranged from 2.3 to 6 years among patients who remained in the trial before being switched to commercial eliglustat or completing the trial.
2 METHODS
ENGAGE (NCT00891202) was a randomized, double-blind, placebo-controlled trial of eliglustat treatment for adults with Gaucher disease type 1 not previously treated with ERT.15 As reported previously, each site's ethics committee or institutional review board approved the protocol, and patients provided written informed consent in accordance with the Declaration of Helsinki. Inclusion and exclusion criteria and laboratory methodology were as previously reported.15 Of note, patients with anemia from causes other than Gaucher disease were excluded based on abnormal folate, iron, vitamin B-12 levels, or the presence of hemoglobin variants to rule out confounding conditions of beta thalassemia or sickle cell disease.
As described previously,15 dose of eliglustat in the trial was determined by plasma levels of eliglustat, not by CYP2D6 metabolizer phenotype, which was found to be the major determinant of drug levels, leading to the current label dosing recommendations.10
The primary efficacy endpoint was the percent change from baseline in spleen volume at 9 months follow-up. Key secondary efficacy endpoints were absolute change in hemoglobin concentration and percent changes in platelet count and liver volume assessed with the use of a prespecified, fixed-sequence, hypothesis-testing approach. An independent core laboratory (BioClinica, Newtown, PA, USA) performed central blinded analysis of all imaging data, including liver and spleen volume, bone mineral density, and bone marrow burden (BMB).15 The primary and secondary endpoints were met in the 9-month double-blind primary analysis period,15 after which patients on placebo switched to eliglustat during the open-label trial extension. Accordingly, for evaluation of longer-term response to eliglustat treatment, analyses were performed by each patient's time on eliglustat rather than by the patient's time in the trial.
Efficacy outcomes were evaluated in all eliglustat-treated patients. In addition, efficacy was separately studied in the subset of patients who were exposed to eliglustat for 4.5 years; this cutoff was chosen because only three patients had data beyond this timepoint. The following efficacy endpoints were evaluated as mean absolute change and percent change with standard error of the mean (SEM) from baseline to 4.5 years of eliglustat treatment: spleen volume in multiples of normal (MN, calculatedby comparison with normal spleen volume, 0.2% bodyweight5), liver volume (MN, calculated by comparison with normal liver volume, 2.5% of bodyweight5), hemoglobin concentration (g/dl), platelet count (×109/L), lumbar spine and femur T-scores and Z-scores,15 total BMB score,22, 23 fatigue severity score, 36-Item Short Form Survey (SF-36), Gaucher disease severity score (DS3), and serum levels of the Gaucher disease biomarkers, chitotriosidase (an established biomarker of Gaucher disease reflecting burden of alternatively activated lipid-laden macrophages), glucosylsphingosine (a secondary accumulating sphingolipid and a highly specific validated biomarker of Gaucher disease),24, 25 glucosylceramide (the primary sphingolipid accumulating in Gaucher disease),24 monosialodihexosylganglioside (GM3, a precursor for more complex gangliosides), and macrophage inflammatory protein (MIP)-1β (a marker of metabolic inflammation and skeletal involvement in Gaucher disease).26 Individual patient responses in these parameters over time were also evaluated. Absolute and percent differences were calculated using individual values for each patient and calculating the mean of these individual changes, rather than subtracting the group final mean from the group baseline mean.
Blood samples for biomarker analysis in plasma (MIP-1β, glucosylceramide, GM3, ceramide, and sphingomyelin) and serum (chitotriosidase) were collected, processed, and stored according to a central laboratory instruction manual. The MIP-1β levels were analyzed by LabCorp Center for Molecular Biology and Pathology (Los Angeles, CA, USA), and levels of glucosylceramide, glucosylsphingosine, GM3, ceramide, and sphingomyelin were analyzed by Genzyme Clinical Specialty Laboratory (Framingham, MA, USA). Chitotriosidase was analyzed by Integrated Genetics (Santa Fe, NM, USA). Chitotriosidase data were normalized based on CHIT1 genotype. Patients who were homozygous for the null variant (24 base-pair duplication in exon 10 of CHIT1) are expected to have no chitotriosidase activity and therefore excluded from the analyses of chitotriosidase over time, whereas patients who were heterozygous for this mutation, and thus have half the normal chitotriosidase activity, had their serum levels normalized by multiplying by two.15
We assessed attainment of therapeutic goals for patients with at least 2.5 years of eliglustat treatment using long-term therapeutic goals established by Pastores et al.27 for patients receiving ERT as follows: reduction of spleen volume ≥50% or a final volume ≤8 MN; reduction of liver volume ≥30% or a final volume ≤1.5 MN; hemoglobin concentration in the non-anemic range (≥11 g/dl for women and ≥12 g/dl for men); and platelet count ≥120 × 109/L if the baseline value was ≥60 × 109/L, or a doubling of count if baseline was <60 × 109/L. Additionally, we assessed response of platelet count according to the revised platelet therapeutic goal of ≥100 × 109/L from the European Working Group on Gaucher Disease (EWGGD).28 For this analysis, the data from the latest timepoint up to 4.5 years were used (i.e., 2.5, 3.5, or 4.5 years). We also assessed therapeutic response stratified by baseline disease severity in the patients with at least 2.5 years of eliglustat treatment, as follows: mild/moderate splenomegaly (spleen volume ≤15 MN) versus severe splenomegaly (spleen volume >15 MN); mild hepatomegaly (liver volume <1.25 MN) versus severe hepatomegaly (liver volume ≥1.25 MN); no/mild/moderate anemia (hemoglobin ≥11 for males; ≥10 g/dl females) versus severe anemia (hemoglobin <11 g/dl males; <10 g/dl females); and no/mild/moderate thrombocytopenia (platelet count ≥60 × 109/L) versus severe thrombocytopenia (platelet count <60 × 109/L). Mean baseline value, mean final value, mean absolute and percent change from baseline to final assessment (2.5, 3.5, or 4.5 years) were determined.
Individual patient trajectories for hematologic and visceral parameters (hemoglobin, platelet count, liver volume, and spleen volume) and Gaucher disease biomarkers (normalized chitotriosidase and glucosylsphingosine) were plotted in patients treated with eliglustat for at least 9 months and up to 4.5 years, along with mean values for hematologic and visceral parameters and median values for biomarkers. For lumbar spine T-score and BMB score, individual values were tabulated. Changes in osteopenia category from baseline to final assessment were evaluated based on baseline osteopenia status. Individual changes in BMB score were assessed by determining the proportion of patients with margins of change greater than or equal to two points and four points.23
Frequency of adverse events, events considered related to eliglustat, seriousness of adverse events, and severity of adverse events were reported for all eliglustat-treated patients during both the primary analysis period and the trial extension period (range of eliglustat exposure: 0.5–6 years). Adverse events, coded based on the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms, considered related to eliglustat treatment that were reported in ≥5% of patients during eliglustat treatment were summarized. For comparison, the number of patients reporting this subset of adverse events while on placebo or eliglustat treatment during the 9-month primary analysis period were also summarized.
3 RESULTS
Of the 40 patients enrolled in the trial, 39 entered the open-label extension period and 34 (85%) remained in the trial until they were switched to commercial treatment in the fall of 2014 (all seven US patients) or until the trial was completed. Of the six patients who actively withdrew from the trial, one patient became pregnant, one patient wished to become pregnant, and four patients (all women) chose to withdraw for unknown reasons not related to adverse events.
The maximum time on eliglustat treatment for patients who remained in the trial until commercial approval of eliglustat in their country or until completion of the trial ranged from 2.3 to 6 years depending on when the patient was randomized (randomization spanned 2 years), whether the patient was randomized to eliglustat or placebo in the primary analysis period, and whether the patient lived in the United States (the first country to grant approval) and was therefore switched to commercial eliglustat before the trial ended (Figure S1 shows individual patient dispositions). By design, the trial extension ended on a calendar date rather than when patients completed a specified interval of time in the trial or time on eliglustat.
Baseline demographics and disease status were similar among the full trial cohort and patients who had or did not have 4.5-year data (Table 1). Overall, the patients with 4.5 years on eliglustat had slightly more severe baseline disease with respect to age at first symptoms and a higher proportion of genotypes associated with more severe disease.
| Parameter | Intent-to-treat population15 (N = 40) | Patients who accrued ≥4.5 years on eliglustat (n = 14) | Patients who did not accrue 4.5 years on eliglustat (n = 26) |
|---|---|---|---|
| Male to female ratio | 20:20 | 8:6 | 12:14 |
| Age at first dose of eliglustat, years ± SD | 31.8 ± 11.3 | 31.1 ± 10.8 | 32.2 ± 11.7 |
| Age at first symptom, years ± SD | 16.0 ± 11.3 | 12.5 ± 11.2 | 18.0 ± 11.2 |
| Age at diagnosis, years ± SD | 21.1 ± 11.5 | 20.1 ± 10.8 | 21.7 ± 12.0 |
| CYP2D6 status, n (%) | |||
| Poor | 0 | 0 | 0 |
| Intermediate | 3 (8) | 1 (7) | 2 (8) |
| Extensive | 36 (90) | 13 (93) | 23 (88) |
| Ultra-rapid | 1 (3) | 0 | 1 (4) |
| Gaucher disease genotype category, n (%) | |||
| L444P/Other (p.Leu483Pro/other) | 4 (10) | 3 (21) | 1 (4) |
| N370S/L444P (p.Asn409Ser/p.Leu483Pro) | 6 (15) | 2 (14) | 4 (15) |
| N370S/N370S (p.Asn409Ser/p.Asn409Ser) | 11 (28) | 1 (7) | 10 (38) |
| N370S/Other (p.Asn409Ser/other) | 16 (40) | 7 (50) | 9 (35) |
| Other/Other | 3 (8) | 1 (7) | 2 (8) |
- Abbreviations: CYP2D6, cytochrome P450 2D6; SD, standard deviation.
3.1 Visceral and hematologic response
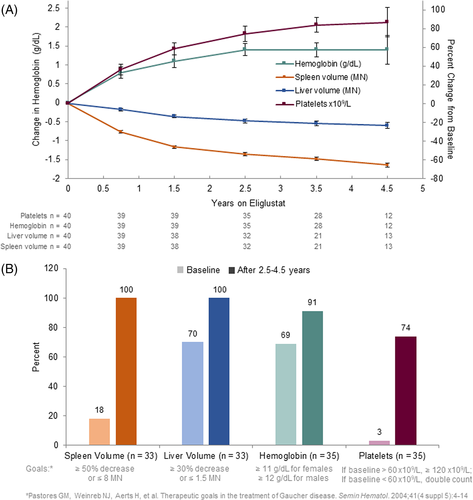
Spleen volume decreased by a mean of 66% from 17.1 MN to 5.8 MN (n = 13) and liver volume decreased by a mean of 23% from 1.5 MN to 1.1 MN (n = 13) after 4.5 years on eliglustat (Figure 1A, Table 2). Individually, all 39 patients with follow-up data had a decrease in spleen volume and either maintained a normal liver volume or had a decrease in liver volume at their final assessment (Figure S2). Among patients with at least 2.5 years on eliglustat (N = 33), the largest margins of improvement were seen among the patients with the largest organ volumes at baseline (>15 MN for spleen and >1.25 MN for liver) (Table S1).
| Parameter | n | Baseline | 4.5 years | Absolute change | Percent change |
|---|---|---|---|---|---|
| Mean spleen volume (MN), (min, max) | 13 | 17.1, 8.6, 28.4 | 5.8, 3.2, 10.0 | −11.4, −21.2, −4.8 | −66%, −75, −53 |
| Mean liver volume (MN), (min, max) | 13 | 1.5, 1.1, 2.2 | 1.1, 0.9, 1.4 | −0.4, −0.8, −0.1 | −23%, −38, −5 |
| Mean hemoglobin (g/dl), (min, max) | 12 | 11.9, 7.9, 14.3 | 13.4, 10.2, 15.4 | 1.4, –0.7, 4.6 | 13.5%, –5, 58 |
| Mean platelet count (×109/L), (min, max) | 12 | 67.6, 40.5, 98.0 | 122.6, 62.0, 189.0 | 55.0, 20.5, 97.5 | 87%, 40, 241 |
| Median chitotriosidase (nmol/h/ml), (min, max) | 11 | 13 394, 2928, 35 106 | 2312, 840, 6346 | −9439, −30 879, −616 | −82%, −92, −21 |
| Median glucosylsphingosine (ng/ml), (min, max) | 10 | 518.5, 170, 1040 | 72.1, 11.1, 273 | −481, −767, −145 | −84%, −98, −74 |
| Median glucosylceramide (μg/ml), (min, max) | 11 | 11.5, 6.1, 17.8 | 2.4, 2.0, 7.4 | −8.6, −14.1, −3.1 | −79%, −83, −37 |
| Median MIP-1β (pg/ml), (min, max) | 8 | 313.8, 108.3, 557.2 | 107.4, 39.8, 169.0 | −256.5, −426.2, −57.9 | −71%, −88, 29 |
| Median GM3 (μg/ml), (min, max) | 6 | 23.5, 15.0, 34.0 | 7.5, 4.0, 16.0 | −16.00, −28.6, −6.0 | −62%, −82, −40 |
| Mean spine T-score, (min, max) | 9 | −1.07, −1.7, 0.6 | −0.53, −1.6, 1.3 | 0.53, –0.2, 1.1 | — |
| Mean BMB score, (min, max) | 10 | 8.4, 6.0, 11.3 | 7.9, 5.5, 10.7 | −0.51, −3.8, 4.0 | — |
| Mean Gaucher disease severity scoring system (DS3) score, (min, max) | 9 | 4.4, 3.4, 6.4 | 2.5, 1.6, 3.4 | −2.0, −3.4, −1.0 | −44.1, −63.6, −22.7 |
- Abbreviations: BMB, bone marrow burden; GM3, monosialodihexosylganglioside; MIP-1B, macrophage inflammatory protein 1 beta; MN, multiples of normal.
After 4.5 years of eliglustat treatment (n = 12), hemoglobin concentration increased by a mean of 1.4 g/dl from 11.9 to 13.4 g/dl and platelet count increased by a mean of 87% from 67.6 to 122.6 × 109/L (Figure 1A, Table 2). Individually, among the 20 men with follow-up data, one had anemia at baseline and none had anemia at their final assessment (Figure S2). Among the 19 women with follow-up data, 11 were anemic at baseline. At the final assessment, four of these women had persistent anemia (with discordant improvements in organomegaly, platelet count, biomarkers, as well as lumbar spine T-score) and no woman had developed new anemia (Figure S2). Individually, all 39 patients with follow-up data had an increase in platelet count, with 26 of 39 maintaining final values ≥120 × 109/L27 and 31 of 39 with final values ≥100 × 109/L28 (Figure S2). The one patient who had an ultrarapid CYP2D6 metabolizer phenotype was among the patients who did not attain a platelet count ≥100 × 109/L; however, this patient had a robust treatment response for all other parameters. Among patients with at least 2.5 years on eliglustat (n = 35), the largest margins of improvement in hemoglobin levels were seen among the patients with severe baseline anemia and thrombocytopenia (Table S1).
Among the 35 patients who completed at least 2.5 years of eliglustat treatment in the trial, the proportion of patients meeting the Pastores et al. long-term therapeutic goals for spleen, liver, hemoglobin, and platelets increased markedly after eliglustat treatment (Figure 1B). With respect to the lower platelet goal threshold of ≥100 × 109/L established by the European Working Group on Gaucher Disease, 8.6% of patients had values above this threshold at baseline compared to 80% of patients after eliglustat treatment. Among the nine patients with severe thrombocytopenia (platelet count <60 × 109/L) at baseline and at least 2.5 years of data on eliglustat, four attained the Pastores et al. platelet therapeutic goal,27 three by achieving a final count >120 × 109/L, and one by doubling the count; the remaining five patients who did not achieve this goal nevertheless had increases in platelet count of 40%–80%.
3.2 Bone response
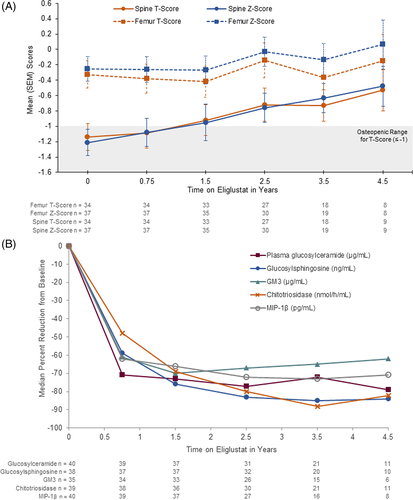
Mean lumbar spine T-score was in the osteopenia range at baseline and improved to the normal range within 1.5 years (Figure 2A). Mean femur T-score was in the normal range at baseline and improved with treatment (Figure 2A). The Z-score changes paralleled T-scores for both spine and femur (Figure 2A). Among the nine patients with 4.5-year data, mean spine T-scores improved from −1.07 at baseline to −0.53 (Table 2). Individual responses among the 34 patients with baseline and follow-up data are shown in Table S2. The T-score increased in 27 patients (79%), remained the same in one patient (3%), and decreased in six patients (18%). All 14 patients with normal baseline values maintained normal values; six of 17 patients with baseline osteopenia attained normal bone density and one additional patient who had no baseline value but whose value at 1.5 years was osteopenic also attained normal bone density; one osteopenic patient progressed to osteoporosis, and both patients with baseline osteoporosis remained osteoporotic.
Total BMB score decreased over time in the majority of patients (Table S3). Among patients with 4.5-year data (n = 10), mean total BMB score improved from 8.4 to 7.9 (Table 2). Among 39 patients with follow-up data, BMB scores decreased in 72% of patients. While there is no established minimal clinically significant change for BMB, nine patients had a decrease ≥4 points (23%), 20 patients had a decrease ≥2 points (51%), 15 had scores that were unchanged or changed by <2 points (38%) and four patients had scores that increased by ≥2 points, including two patients who had a score that increased by ≥4 points (Table S3). No patient who had an increase in BMB score also had a decrease in T-score.
No patient on eliglustat experienced a bone crisis. The one bone crisis that occurred during the trial was during the primary analysis in a patient receiving placebo.
3.3 Biomarker response
Plasma chitotriosidase, glucosylsphingosine, glucosylceramide, GM3, and MIP-1β decreased with treatment in all patients (Figure 2B and Table 2). Normalized chitotriosidase activity was elevated in all patients at baseline, with a median value 96-fold above the upper limit of normal (ULN). The median percent decrease was 48% after 9 months (n = 38) and 82% after 4.5 years (n = 11). Serum glucosylsphingosine values were elevated in all patients at baseline, with a median value 61-fold above the ULN. Among patients with follow-up data, median decrease in serum glucosylsphingosine levels was 59% after 9 months (n = 37) and 84% after 4.5 years (n = 10). Serum glucosylceramide levels were elevated in most patients at baseline, with a median value 1.6-fold above the ULN. Individual patient trajectories for chitotriosidase and glucosylsphingosine are shown in Figure S3. Median serum concentration of glucosylceramide decreased to normal within 9 months and remained in the normal range for the duration of the trial, representing an overall median decrease of 79% at 4.5 years. Serum GM3 concentration was elevated in most patients at baseline, with a median value 1.4-fold above the ULN. Median GM3 levels normalized by 9 months (n = 34) and remained in the normal range, representing an overall median decrease of 62% after 4.5 years (n = 6). Serum MIP-1β levels were elevated in all patients at baseline with median three-fold elevation above the ULN; median values fell to near normal levels after 9 months (n = 39) and normalized by 4.5 years of eliglustat therapy (n = 8). Plasma sphingomyelin and ceramide levels were in the normal range at baseline and remained in the normal range throughout the trial (data not shown).
3.4 Quality of life
The mean fatigue severity score and SF-36 physical and mental component scores were in the normal range throughout the study (data not shown). Among the subset of nine patients with baseline and 4.5-year follow-up data for Gaucher disease severity score, the mean score improved from 4.4 (moderate range) at baseline to 2.5 (borderline to mild range) after 4.5 years of eliglustat (Table 2).
3.5 Safety
Safety data in the ENGAGE trial represent 155 patient-years of eliglustat exposure. During up to 6 years of treatment, 90% of patients experienced at least one adverse event and 55% of patients experienced at least one adverse event considered related to eliglustat treatment. As the trial progressed, the proportion of patients reporting any adverse event or any related adverse event decreased substantially, as did the number of overall and related adverse events per 100 patient-years.12 By event, 99% of adverse events were mild or moderate and 83% were considered unrelated to eliglustat. There were no deaths in the trial and no patients withdrew due to adverse events. Five patients experienced a total of seven serious adverse events, all of which were mild or moderate. As reported previously,20 two events of mild atrioventricular block in one patient were considered serious and related to eliglustat but did not lead to trial discontinuation. The additional serious adverse events (all unrelated, occurring once per patient, none resulting in trial discontinuation) were appendicitis (mild), acute appendicitis (moderate), biliary colic (mild) related to gallstones, osteoarthritis (moderate) leading to hip replacement, and non-sustained ventricular tachycardia (mild; same patient who also had mild atrioventricular block).
Nine treatment-related adverse events were reported in 5% or more patients during the entire trial (Table 3). The most frequently reported treatment-related adverse event, headache, was reported in 10% of patients overall. Five of the nine most common treatment-related events (headache, dizziness, diarrhea, abdominal distension, and abdominal pain) during eliglustat treatment (155 patient-years of eliglustat exposure) were also reported as treatment-related in an equal or greater proportion of placebo-treated patients during the 9-month primary analysis period (15 patient-years of exposure to placebo).
| All patients during eliglustat treatment (N = 40) | 9-month primary analysis | ||
|---|---|---|---|
| Placebo N = 20 | Eliglustat N = 20 | ||
| Total exposure to eliglustat, patient-years | 155 | 15 | 15 |
| Mean duration of treatment per patient, years | 3.87 ± 1.26 | 0.76 ± 0.05 | 0.77 ± 0.075 |
| Adverse events, n (%) | |||
| Headache | 4 (10) | 3 (15) | 1 (5) |
| Abdominal distension | 3 (7.5) | 1 (5) | 0 |
| Dyspepsia | 3 (7.5) | 0 | 0 |
| Diarrhea | 2 (5) | 4 (20) | 2 (10) |
| Abdominal pain | 2 (5) | 2 (10) | 1 (5) |
| Dizziness | 2 (5) | 2 (10) | 0 |
| Atrioventricular block second degree | 2 (5) | 0 | 0 |
| Dry mouth | 2 (5) | 0 | 0 |
| Nausea | 2 (5) | 0 | 1 (5) |
4 DISCUSSION
Treatment with eliglustat for up to 4.5 years in previously untreated adults with Gaucher disease type 1 in the Phase 3 ENGAGE trial resulted in continued improvement of hematologic, visceral and bone manifestations with commensurate reduction of bioactive lipids and other inflammatory biomarkers. These results are consistent with results observed after 4 years and 8 years of eliglustat treatment in the Phase 2 trial of previously untreated patients.19 Those with the greatest baseline burden of disease (i.e., lowest platelet count and hemoglobin concentrations and highest spleen and liver volumes) experienced the largest improvements (Table S1). Moreover, the favorable safety and tolerability profile of eliglustat in this trial is consistent with what has been reported among treatment-naïve patients during 8 years in the Phase 2 trial,18 ERT switch patients during 4 years in the Phase 3 ENCORE trial,21 and mostly ERT switch patients in the Phase 3 EDGE trial of once-daily versus twice-daily eliglustat dosing.16 Collectively, these trials represent a total of 1400 patient-years of eliglustat exposure.12
The rarity and heterogeneity of Gaucher disease present prohibitive challenges in conducting trials with large number of patients and including a comparator arm. That notwithstanding, the placebo-controlled ENGAGE trial met primary and secondary endpoints in the primary analysis period, and individual patient responses to eliglustat show that most patients had improvements in all or most disease manifestations. These results are consistent with results of the Phase 2 open-label trial of treatment-naïve patients. No patient experienced clinical decline, although a small subset of patients did not improve with respect to platelet or hemoglobin values (Figure S2).
Ultimately, >90% of patients achieved therapeutic goals for spleen volume, liver volume, and hemoglobin. Persistent thrombocytopenia or lack of platelet response has been reported in some non-splenectomized patients with severe baseline thrombocytopenia after 4–10 years of ERT,29-31 and has been attributed to advanced splenic disease associated with infarcts and focal deposits.32 In the ENGAGE trial, platelet counts increased with eliglustat treatment in all patients, and the platelet response to eliglustat was in keeping with the EWGGD consensus guideline goal (≥100 × 109/L).28 Although hemoglobin levels increased in almost all patients, four women had persistent anemia despite having concomitant clinically meaningful improvements in spleen volume, liver volume, platelet count, biomarkers, and lumbar spine T-scores. As per the trial protocol, all of these women had screened negative for hemoglobin variants at baseline to exclude beta thalassemia or sickle cell disease; however, screening for causes of anemia other than Gaucher disease did not necessarily exclude autoimmune hemolytic anemia, which could have developed after screening and is more common in Gaucher patients than in the general population.33 In addition, women with persistent anemia could have had inadequate iron stores due to elevated ferritin levels.34
Gaucher disease pathophysiology, beyond deficiency of acid β-glucosidase and lysosomal accumulation of glucosylceramide, is complex involving alternative metabolism of substrate via acid ceramidase that generates glucosylsphingosine and system-wide involvement beyond the macrophage.35, 36 The suite of biomarkers evaluated in ENGAGE was designed to capture this multidimensional pathophysiology that underlies disease manifestations. First, the baseline elevated plasma levels of primary and secondary substrates (glucosylceramide and glucosylsphingosine) were strikingly reduced with eliglustat treatment. Concomitantly, eliglustat resulted in dramatic reduction of chitotriosidase, a marker of the burden of alternatively activated lipid-engorged Gaucher macrophages. Moreover, another macrophage biomarker of Gaucher disease, MIP-1β, which has been associated with skeletal disease, was also reduced with eliglustat treatment.26 The pattern of biomarker responses in the ENGAGE trial strongly parallels responses in the smaller, Phase 2, open-label study in more severely affected treatment-naïve patients. Marked reductions were observed in the first 9 to 18 months of treatment in both studies, with smaller incremental responses out to 4.5 years in ENGAGE and 8 years in the Phase 2 trial.18
The aforementioned constellation of biomarker responses reflect amelioration of Gaucher disease pathophysiology and underlie the clinical responses elicited by eliglustat therapy in the ENGAGE trial, vis-à-vis decrease of splenomegaly, hepatomegaly, thrombocytopenia, and anemia. Reduction of lipid-laden macrophage storage cells and associated inflammatory pathology is reflected by reduction of organomegaly and marrow infiltration and cytopenia, thereof.37-39 Bone mineral density improved, with mean lumbar spine T-scores moving from the osteopenic range at baseline to the normal range after 1.5 years of eliglustat treatment, with continuing improvement over the duration of the trial (Figure 2A). Although mean improvement in BMB score was modest and there is no established clinically significant degree of change, 18 of 22 patients with baseline scores of 10 or higher had at least a two-point improvement (Table S3), with only one of these patients (who had data going out to only 1.5 years) showing a small increase (0.16). Finally, it is notable that no patient had a bone crisis while receiving eliglustat; the only bone crisis that occurred during the trial was in a patient on placebo during the primary analysis period.15 Interestingly, glucosylsphingosine, which is a known osteoblastic toxin,36 was markedly reduced by eliglustat therapy. Of note, GM3, which plays a role in osteoclast differentiation,40 was also markedly reduced with eliglustat therapy.
Taken together, multidimensional responses with eliglustat monotherapy in the ENGAGE trial underscore the key role of glucosylceramide synthase in Gaucher disease pathophysiology and as a therapeutic target. Not only does eliglustat appear to alleviate the substrate accumulation caused by insufficient catalytic activity of mutant glucocerebrosidase, it also seems to abrogate increased synthesis of the substrate triggered by metabolic inflammation and possible accompanying complement activation.3 Importantly, plasma concentrations of ceramide and sphingomyelin remained within normal ranges.
For previously untreated patients who participated in the Phase 3 ENGAGE clinical trial and open-label extension and received eliglustat treatment for up to 4.5 years, the significant improvements in hematologic, visceral, biomarker, and bone disease parameters in the first 9 months of treatment were maintained or further improved with continued treatment. Together with the Phase 2 trial in treatment-naive patients18 and real-world evidence from the International Collaborative Gaucher Group (ICGG) Gaucher Registry,41 this study supports the use of eliglustat as first-line treatment for previously untreated patients with GD1 without the need for “debulking” with ERT.
ACKNOWLEDGMENTS
The ENGAGE Trial was sponsored by Sanofi Genzyme. The authors thank the participating patients, who were compensated for travel expenses, and health care professionals, whose institutions received funding for clinical trial-related expenses from Sanofi Genzyme. We also thank Laurie LaRusso, MS, ELS, of Chestnut Communications for medical writing support funded by Sanofi Genzyme, and Lisa Underhill, MS (Director of Scientific Communications and Publications for eliglustat, Sanofi Genzyme Global Medical Affairs) for writing assistance and critical review of the manuscript.
CONFLICT OF INTEREST
Pramod Mistry: Lead principal investigator in the eliglustat ENGAGE trial and principal investigator in the eliglustat ENCORE trial. Member of the International Collaborative Gaucher Group (ICGG) Gaucher Registry North American Advisory Board. Receives research support from Sanofi Genzyme and honoraria and travel reimbursement from Sanofi Genzyme. Elena Lukina: Lead principal investigator in the eliglustat Phase 2 trial; principal investigator in the eliglustat ENGAGE, ENCORE, and EDGE trials. Has received honoraria and travel reimbursement from Sanofi Genzyme and Shire. Hadhami Ben Turkia: Principal investigator in the eliglustat ENCORE trial. Suma P. Shankar: Principal investigator in the eliglustat ENGAGE trial. Site primary investigator in clinical trials and received research support and educational grants from Sanofi Genzyme, Shire, Protalix, Actelion, and Amicus. Hagit Baris Feldman: Principal investigator in the eliglustat ENGAGE trial. Recipient of research grants from Pfizer, Sanofi Genzyme, Shire, of honoraria from Sanofi Genzyme and Shire, of travel grants from Genzyme, Shire, Protalix and Pfizer and advisory board member of Sanofi Genzyme and Shire. Marwan Ghosn: Principal investigator in the eliglustat ENGAGE trial. Atul Mehta: Principal investigator in the eliglustat ENGAGE trial. Has received honoraria and travel reimbursement from Sanofi Genzyme. Seymour Packman: Principal investigator in the eliglustat ENGAGE trial. Received research and programmatic support from Sanofi Genzyme, Shire HGT Corporation, Amicus Corporation, Actelion Corporation, and BioMarin Pharmaceutical. Member of the speaker's bureaus of Shire and Sanofi Genzyme. Heather Lau: Principal investigator in the eliglustat ENGAGE trial. Has received honoraria and travel reimbursement from Sanofi Genzyme. Milan Petakov: Principal investigator in the eliglustat ENGAGE trial. Has received honoraria and travel reimbursement from Sanofi Genzyme. Sarit Assouline: Principal investigator in the eliglustat ENGAGE trial. Manisha Balwani: Principal investigator in the eliglustat ENGAGE and ENCORE trials. Member of the ICGG Gaucher Registry North American Advisory Board. Has received honoraria and travel reimbursement from Sanofi Genzyme. Sumita Danda: Principal investigator in the eliglustat ENGAGE trial. Evgueniy Hadjiev: Principal investigator in the eliglustat ENGAGE trial. Has received honoraria and travel reimbursement from Sanofi Genzyme. Andres Ortega: Principal investigator in the eliglustat ENGAGE trial. Meredith C. Foster: Employee of Sanofi Genzyme. Sebastiaan J.M. Gaemers: Employee of Sanofi Genzyme and holds Sanofi stock. M. Judith Peterschmitt: Employee of Sanofi Genzyme and holds Sanofi stock.
Open Research
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to patient-level data and related documents. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.




