Clinical and biological characteristics of leukemia cutis in chronic lymphocytic leukemia: A study of the French innovative leukemia organization (FILO)
Grégory Lazarian and Michaël Munger are these authors contributed equally
Patients with chronic lymphocytic leukemia (CLL) exhibit a variety of skin lesions including mostly non-specific cutaneous manifestations (such as cutaneous infections or exaggerated reactions to insect bite) and secondary cutaneous malignancies, as patients are at high risk of developing basal cell carcinoma, squamous cell carcinoma, melanoma, and Merkel cell carcinoma. Specific cutaneous infiltration by neoplastic B lymphocytes with clinically identifiable skin lesions, also called leukemia cutis (LC), is more uncommon and has seldom been reported in chronic lymphocytic leukemia.1, 2 While CLL is usually restricted to blood, lymph nodes, spleen and bone marrow, the origin of such cutaneous presentation is elusive. In CLL, a number of recurrent gene mutations have been described and support the clinical heterogeneity of the disease.3 However, the association of these mutations and LC has not been systematically investigated.
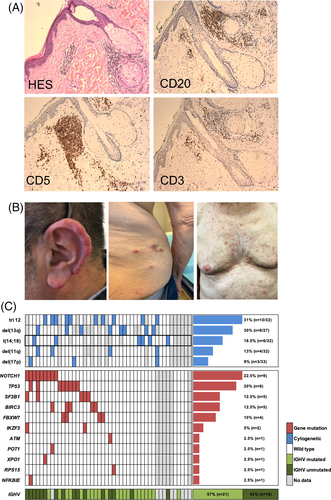
To gain insight into the genetic background of LC-CLL and a potential link with cutaneous infiltration, we retrospectively analyzed the clinical and molecular features of 46 patients with specific LC from centers of the French Innovative Leukemia Organization (FILO). To date, this is the largest cohort of CLL patients with LC with genetic characterization. The specific skin involvement by CLL was proven by biopsy for all patients included in the study (Figure 1(A)).
We collected clinical data, peripheral blood cytogenetics assessed by FISH and conventional karyotype, immunoglobulin heavy chain gene (IGHV) mutational status and results of targeted next-generation sequencing (NGS) of genes frequently mutated in CLL, including TP53 (exon 2–11), NOTCH1 (exon 34-3′UTR), ATM (full gene), FBXW7 (exon 8–11), SF3B1 (exon 14–46), POT1 (exon 5–10), XPO1 (exon 15), BTK (exon 15), PLCG2 (exon 19, 20, 24), RPS15 (exon 4) and IKZF3 (exon 5). We identified 46 patients with LC-CLL [sex ratio = 0.45; median age 61.2 years (43–85)]. All patients were diagnosed with CLL prior to LC. Median time between skin manifestations and CLL was 5.4 years (1–18 years). The majority of patients were at an early stage of CLL when cutaneous lesions occurred (Binet A: 55%, Binet B: 29%, Binet C: 14%), but two patients presented with Richter's transformation. Patient and clinical characteristics are summarized in Table S1.
A complete description of the skin manifestation was available in 38 cases. Skin lesions were either diffuse (n = 16), localized (n = 13) or regional (n = 9). Head and neck were the most common site (22 cases) with a high incidence of earlobe lesions (10/38 cases, 26%), while lesions on arms and legs, trunk and extremities were found in 17, 13 and 14 cases respectively. The high incidence of auricular manifestations was consistent with previously published case reports describing similar swelling, non-tender, violaceous lesions involving earlobes, tragus or helix, either symmetric or asymmetric4 (Figure 1(B)). Although the underlying mechanism explaining the infiltration at this predilection site is currently unknown, the cartilaginous nature of the tissue may be a favoring factor. Lesions involving the nostrils or the tip of the nose were observed in three patients as well, suggesting a similar phenomenon.
Regarding the appearance of the lesions, the most common skin manifestations were papular and/or nodular (n = 24) and patches (n = 9). Plaques, vesicles, mosquito bite-like, rhinophyma-like, ulcerations or necrotic lesions were uncommon. Some infiltrates appeared at the sites of previous scars following herpes, mosquito bite or plant wound in four patients, in line with reports of lesions developing at the previously affected healed sites of herpes zoster, herpes simplex or Borrelia burgdorferi.5 In these cases, it is unclear whether CLL cells are recruited to the skin in response to an infectious stimulus or strictly metastatic.
Assessment of the IGH gene clonality in 12 skin biopsies retrieved an identical VDJ rearrangement as compared to the matched peripheral blood CLL cells, indicating a clonal relation between the tumoral cells in the two sites. Furthermore, IGHV mutational status was available for 37 patients. The majority of cases were IGHV mutated (n = 21/37, 57%) (Figure 1(C)). It is noteworthy that the IGHV4-34 gene ranked among the most frequent IGHV genes (8/37, 21.6%), whereas it usually account for 10% of CLL, suggesting a potential role of an antigenic stimulation occurring at the site of infiltration.6
Conventional karyotype was performed in 32 patients. It was normal in seven cases and complex (>3 abnormalities) in six cases. An intriguing finding was the unexpected high frequency of t(14;18) translocation in 6/32 patients (18.5%). This translocation, involving the immunoglobulin heavy chain (IGH) and BCL2 loci, usually occurs in less than 5% of CLL patients. Interestingly, a case of cutaneous infiltrate of CLL with t(14;18) at a site of Borrelia infection was reported.7 Among the cases tested by FISH, trisomy 12 was frequent (10/32; 31%), while 13q, 11q and 17p deletions were respectively found in 30% (8/27), 13% (4/32) and 9% (3/33) of patients (Figure 1(C)).
The mutational profile of the peripheral blood mononuclear cells was available for 40 patients. Fifteen patients (37%) had no mutation while 15 (37%) had one single mutation and 10 patients (25%) harbored two to five mutated genes. The most frequently mutated genes were NOTCH1 (22.5%), TP53 (20%), SF3B1 (12.5%) and BIRC3 (12.5%). When considering the NOTCH pathway, both FBXW7 and NOTCH1 mutations accounted for 32.5% of patients (Figure 1(C)).
Follow-up data were obtained for 32 patients. Skin lesions tended to occur in Binet stage A and before initiation of any treatment: 20 patients (62.5%) had skin lesions before the first line of treatment, while 12 (37.5%) had received at least one line of treatment before any skin manifestations. Six patients had skin lesions appearing later in the course of the disease. Thirty-one patients received treatment after the development of skin lesions. Among these patients, 13 received a fludarabine or bendamustine-based regimen, five chlorambucil based, five ibrutinib, four polychemotherapy, two venetoclax, one obinutuzumab and one idelalisib. The treatment was initiated because of the presence of skin lesions in 15 patients. The systemic treatment led to a complete skin response in 74% (23/31) of patients, further confirming the clonal relation between skin lesions and hematological disease. The eight patients who did not experience a complete skin response, among which the five patients treated with chlorambucil, were not in complete response at the systemic level either. Among responders, relapses at skin sites were very uncommon, occurring in only two patients, both of whom had received chlorambucil. In the literature, data on the prognostic significance of cutaneous infiltration with CLL are sparse. In a retrospective review of 42 patients, LC did not affect prognosis of patients, the unfavorable prognosis of LC being attributed to Richter transformation instead.2 Altogether, these results suggest that CLL with cutaneous lesions is not associated with poor prognostic characteristics and respond well to CLL systemic treatment.
Extranodal localizations are uncommon in CLL. We recently reported a series of CLL cases with symptomatic bronchial involvement, but the pathogenesis underlying the infiltration of extranodal localization is undefined.8 CLL cells homing to skin could be linked to the expression of chemokine receptors and adhesion molecules, including the upregulation of intercellular adhesion molecule 1 (ICAM-1) and lymphocyte function-associated antigen 1 (LFA-1).9 On the other hand, one third of the patients in our series had a mutation causing an aberration of NOTCH1 signaling. Overactivation of this pathway has been shown to promote the migration of CLL cells through the modulation of the CCR7 receptor.10 Therefore, this abnormality could potentially promote the development of leukemia cutis. Moreover, given that the skin is a peripheral organ of the immune system with associated lymphoid tissue, it provides an optimal microenvironment and conditions for the immune response and represents a potential site for infiltration of the tumoral cells.
In our cohort, the proportion of patients with an IGH-BCL2 rearrangement (6/32 tested by karyotyping) is significantly higher than expected from literature reports.11 No variant translocations t(2;18) (p11;q21) or t(18;22)(q21;q11) involving respectively IGK and IGL loci were observed in our series. The clinical significance of these translocations is controversial but IGH rearrangements involving BCL2 partner are usually not associated with adverse prognosis markers or with unfavorable outcome.11, 12
This rearrangement is a hallmark of follicular lymphoma (FL) but interestingly, skin involvement rarely occurs in FL (about 4% of patients), suggesting that this translocation is not directly responsible for skin infiltration. Besides, while the majority of nodal forms of FL have a t(14;18) or BCL2 rearrangement, these are rare in primary cutaneous follicle center lymphoma (PCFCL), an entity that typically primarily affects the skin. Consequently, the high proportion of this cytogenetic alteration in our cohort is very unexpected. Although it seems significant, its implication in pathogenesis is currently not understood.
In summary, we report here the largest cohort of CLL patients with leukemia cutis. We did not find a specific clinical or biological profile associated with LC and no specific prognostic impact. We found a high frequency of NOTCH1 mutations and unexplained enrichment of t(14;18) that we are currently investigating. Skin lesions occur at an early stage leading, to treatment initiation in half of patients with frequent complete response using CLL systemic treatment.
ACKNOWLEDGMENTS
The authors thank the French Innovative Leukemia Organization (FILO).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
G.L., M.M., F.C. designed the study; G.L., M.M., F.C. analyzed the data; A.Q., M.S.D., L.V., D.L.P., R.G., A.L.P., M.H., F.M., J.P.V., A.B., A.W.R., J.B., A.L., L.M., K.L., B.H., C.F., B.C., L.Y., E.D.N., L.W., A.C., D.R.W., S.P., E.M., A.M., P.F., A.D. and F.B.M. provided clinical samples and data; G.L., M.M., F.C. wrote the paper.
ETHICS STATEMENT
The study was conducted in accordance with national ethical recommendations and the Declaration of Helsinki and approved by the institutional review board of Avicenne Hospital where the data and samples were centralized.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request




