Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation
Shuangyou Liu and Chunrong Tong contributed equally to this work as corresponding authors.
Funding information: Gaobo Healthcare Group, Grant/Award Number: GBHG-CR2017003; the National Key Basic Research Program of China, Grant/Award Number: 2016YFC1303403
Abstract
The prognosis of relapsed acute lymphoblastic leukemia (ALL) after allogeneic transplantation is dismal when treated with conventional approaches. While single-target CD19 or CD22 chimeric antigen receptor (CAR) T-cell therapy has achieved high complete remission (CR) rates in refractory/relapsed B-ALL, it could not maintain a durable remission in most patients. To prolong relapse-free survival, we sequentially combined CD19 and CD22 CAR-T cells to treat post-transplant relapsed B-ALL patients with both CD19/CD22 antigen expression on lymphoblasts. Patient-derived donor cells were collected to produce CAR-T cells that were transfected by lentiviral vectors encoding second generation CARs composed of CD3ζ and 4–1BB. The second T-cell infusion was scheduled at least 1 month, and usually within 6 months after the first CAR-T treatment. Twenty-seven adult and pediatric patients, including 11 (41%) with extramedullary diseases (EMD), received the first CD19 CAR-T and 23 (85%) achieved CR. Subsequently, 21 out of 27 patients received the second CD22 CAR-T and were followed-up for a median of 19.7 (range, 5.6–27.3) months; 14 cases remained in CR, seven relapsed and two of them died from disease progression; Kaplan–Meier survival analysis showed overall survival and event-free survival rates of 88.5% and 67.5%, respectively, at both 12 months and 18 months. CAR-T associated graft-versus-host disease (GVHD) occurred in 23% of patients, with 8% new-onset acute GVHD and 15% persistent or worsened pre-existing cGVHD before CAR-T. This combination strategy of sequential CD19 and CD22 CAR-T therapy significantly improved the long-term survival in B-ALL patients who relapsed after transplantation.
1 INTRODUCTION
With conventional therapies including intensive chemotherapy, donor lymphocyte infusion (DLI) and second transplantation, the prognosis of relapsed acute lymphoblastic leukemia (ALL) after allogeneic hematopoietic cell transplantation (allo-HCT) remains extremely poor.1-4 A large-scale study in adults revealed that the median overall survival (OS) in relapsed ALL patients post-HCT was 5.5 months and estimated 1-year, 2-year and 5-year survival rates were 30 ± 2%, 16 ± 2% and 8 ± 1%, respectively.3 Similar results were observed in children, an investigation among 121 pediatric ALL patients showed a 15% OS rate at 3 years.4
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 antigen has been demonstrated as a potent approach for treating refractory/relapsed (r/r) B-ALL.5-9 However, while single-target CAR-T therapy has shown significant efficacy, it has not been able to maintain a durable remission in most patients. CD19 CAR-T treatment has been reported to achieve a 6-month event-free survival (EFS) rate of 67% and OS rate of 78% among both children and adults.5 A long-term follow-up study in adults displayed a median EFS of 6.1 months and OS of 12.9 months, after 20 months, there was less than 40% EFS in any group of patients.9 Another research project with children and young adults showed EFS rates of 73% at 6 months and 50% at 12 months.7 An alternative target for CAR-T treatment is CD22 antigen, which is also expressed on most B-ALL cells. Recently, CD22 CAR-T immunotherapy has achieved similar anti-leukemic effects in r/r B-ALL patients including those who previously received CD19 CAR-T cells and with dim/negative CD19 expression, resulting in complete remission (CR) rates of 73–80%,10, 11 and median remission duration of 6 months.10
To prolong relapse-free survival, we sequentially combined CD19 and CD22 specific CAR-T cells targeting two different antigens of leukemic blasts to treat post-transplant relapsed B-ALL patients. Here, we report the treatment outcomes of this phase I trial at 19.7-month median follow-up.
2 METHODS
2.1 Patients
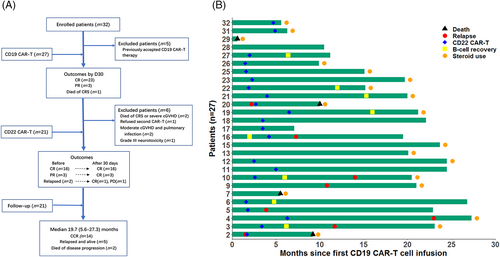
From December 2017 through October 2019, 32 adult and pediatric B-ALL patients relapsing after allo-HCT were enrolled. All patients had both CD19 and CD22 antigen expression on lymphoblasts. Expression of antigens was determined by multiparameter flow cytometry (FCM) in patients with bone marrow involvement. In patients with isolated extramedullary diseases (EMD), blasts for FCM assay were collected from fresh biopsy tissues or cerebrospinal fluid (CSF) (Figure S1(A)), or antigens were detected by immunohistochemical staining. Minimal residual disease (MRD) relapse was not included. Patients with leukemic cells in CSF were eligible if they had no intracranial lesions and less than 20/μl blasts in CSF before CAR-T cell infusion. Patients with grade I acute graft-versus-host disease (aGVHD) or mild chronic GVHD (cGVHD) were eligible. Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2 and adequate organ function were required (inclusion and exclusion criteria in supplemental methods). Five patients who accepted prior CD19 CAR-T therapy were excluded from this article, 27 patients received the first CD19 CAR-T and 21 out of the 27 received the second CD22 CAR-T treatment (Figure 1(A)). The first cell infusion was performed between December 2017 and October 2019, the cut-off date for follow-up was March 31, 2020.
2.2 CAR-T cell treatments
Two rounds of CAR-T therapy were planned for eligible patients, first with murinized CD19 CAR-T cells, followed by humanized CD22 CAR-T cells. The second T-cell infusion was scheduled at least one month after first CAR-T when cytokine release syndrome (CRS) disappeared and cytokines returned to normal, the latest time was usually within 6 months before possible relapse. The time interval between two infusions was based on patients' clinical conditions. For relapsed patients, the second CAR-T was given as early as possible; for partial remission (PR) cases or CR cases with EMD, the second CAR-T was generally administrated within 3 months. Other rules for second infusion included undetectable CAR-T cells (not requisite), no infection, no GVHD or grade I aGVHD or mild cGVHD.
Both CD19 and CD22 CAR vectors used to prepare CAR-T cells in this study have been described before.8, 11 Briefly, they are both lentiviral vectors carrying a second generation CAR with 4–1BB co-stimulatory and CD3ζ signaling domains. The antigen recognition domains of CD19 and CD22 specific CARs are single chain variable fragments (scFvs) obtained from the murine monoclonal antibody FMC63 (CD19), or from a human antibody phage display library (CD22). Patient-derived donor cells were collected for producing CAR-T cells, which were transfected by lentiviral vectors and cultured for 5–8 days. Cryopreserved cells from first leukapheresis were used to manufacture CD22 CAR-T products in 18 patients and a second apheresis was conducted in three cases before second CAR-T due to insufficient cells. All patients received lymphodepleting agent fludarabine (30 mg/m2/day), with or without cyclophosphamide (250 mg/m2/day), on days −5, −4, and −3 prior to each infusion. Patients not using cyclophosphamide included those with peripheral white blood count (WBC) less than 1.0 × 109/L, mild infection or mild organ dysfunction, and a few cases receiving other chemodrugs. Patients with higher tumor burden or disease progression accepted additional short-term and non-intensive chemodrugs before or together with lymphodepleting agents (14 cases in the first CAR-T and two cases in the second CAR-T, details in Table S5) to reduce the disease burden. Intracranial chemotherapy was given to patients with central nervous system leukemia (CNSL), steroids or immunosuppressive agents were allowed for patients with GVHD, and tyrosine kinase inhibitors (TKIs) were allowed for Ph + patients. These medications were withdrawn at least 1–2 days before apheresis and before CAR-T cell infusion. The TKIs were not used within 1 month after CAR-T cell infusion. The construction of CD19 and CD22 specific CARs, CAR-T cell manufactures and clinical protocols are shown in our previous work.8, 11
This study was approved by Beijing Boren Hospital institutional review board and registered on the Chinese Clinical Trial Registry/WHO International Clinical Trial Registry (ClinicalTrials#: ChiCTR-ONC-17013648), written informed consents were obtained in accordance with the Declaration of Helsinki.
2.3 Clinical and laboratory assessments
Disease status and treatment responses were defined according to the guidelines of the National Comprehensive Cancer Network (NCCN).12 Extramedullary diseases were examined by PET-CT or CT/MRI/Ultrasonography. Cytokine release syndrome was graded by the Penn grading scale13 and neurotoxicity was assessed by CTCAE 5.0 version. Graft-versus-host disease was staged and graded in accordance with criteria from Mount Sinai and National Institutes of Health.14, 15 Minimal residual disease was detected by FCM for all patients and real-time quantitative polymerase chain reaction (qPCR) for patients with fusion genes, the MRD level below 1 × 10−4 (both FCM and qPCR) was defined as negative. CAR-T cell numbers were assayed through flow cytometric quantitation of FITC+CD3+ T cells. B-cell aplasia (BCA) was defined as less than 3% CD19/CD22 positive lymphocytes in BM. Cytokines were detected by enzyme-linked immunosorbent assay (ELISA) (details in supplemental methods).
Treatment effects were assessed on Day 30 after each T-cell infusion. The follow-up evaluation was performed every month before the second CAR-T, and every 2–3 months (within a year) or every 6 months (beyond a year) after second CAR-T. Overall survival was defined as the time from the date of first CAR-T infusion to the date of death or last contact in living patients, event-free survival was defined as the time from the date of first or second CAR-T infusion to the date of relapse or death, or last contact in CR patients.
2.4 Statistical analysis
Data graphing and analysis was by R software version 3.6.2 and GraphPad Prism 7. The comparison of means of cytokines and CAR-T cell numbers was performed using paired t test or Wilcoxon test, categorical variables were compared by the chi-square or Fisher exact test. A p value <0.05 was considered to be statistically significant. The probabilities of OS and EFS were estimated by the Kaplan–Meier method. The time-to-event (death, relapse or survival) analyses were calculated from the date of first CAR-T infusion (EFS in patients achieving CR after second CD22 CAR-T was calculated from the date of second infusion) to the date of death, relapse or last follow-up.
3 RESULTS
3.1 Patient characteristics
The baseline information and characteristics of 27 patients at enrollment were summarized in Table S1 and Table 1(A). The median age was 21 (range, 1.6–55) years, including 18 (67%) adults and nine (33%) children younger than 18 years old. All patients had one prior allo-HCT, of them, 20 (74%) accepted hematopoietic stem cells from HLA-haploidentical family members, six from matched siblings or unrelated donors and one from 5/6 HLA-matched unrelated cord blood. Sixteen (59%) cases showed isolated hematologic relapse and blast cells varying between 11–91%; 11 (41%) patients relapsed with EMD (EMD only, n = 4; both EMD and BM, n = 7), six were single-site involvement and five had multifocal diseases (defined as ≥ two sites of EMD). High-risk cytogenetic changes included complex karyotype (n = 7), BCR/ABL rearrangement (n = 5, three were T315I mutated) and MLL/AF4 fusion gene (n = 1). Four patients presented with mild cGVHD. No patients had a history of CD19 or CD22-directed antibody (blinatumomab or inotuzumab) treatment.
| (A) Baseline characteristics | ||
|---|---|---|
| Characteristics | No. (n = 27) | % of patients |
| Age (years) | ||
| Median | 21 (range, 1.6–55) | |
| Children (<18) | 9 | 33 |
| Adults | 18 | 67 |
| Sex | ||
| Male | 14 | 52 |
| Female | 13 | 48 |
| Type of transplant | ||
| HID | 20 | 74 |
| MSD | 3 | 11 |
| MUD | 3 | 11 |
| UCB | 1 | 4 |
| Duration from HCT to relapse (months) | ||
| Median | 9 (range, 3–31) | |
| <6 | 8 | 30 |
| 6–12 | 11 | 40 |
| >12 | 8 | 30 |
| Disease status at enrollmenta | ||
| Isolated BM (blasts 11–91%) | 16 | 59 |
| Isolated EMDb | 4 | 15 |
| Combined BM and EMD | 7 | 26 |
| EMD distribution | ||
| Overall | 11 | 41 |
| Multiple sitesc | 5 (1 with CNSL) | 19 |
| Single site | 6 | 22 |
| CNS | 4 | |
| Others (breast, mediastinum) | 2 | |
| High-risk cytogenetic changes | ||
| Complex karyotype | 7 | 26 |
| Fusion gene | 6 | 22 |
| BCR/ABL | 5 (three with T315I mutation) | |
| MLL/AF4 | 1 | |
| Treatment after relapse | ||
| Overall | 15 | 55 |
| CT or +TKI | 9 | 33 |
| DLI or +CT | 3 | 11 |
| Others (IFN, HSC, or +CT) | 3 | 11 |
| (B) CAR-T therapy, toxicity and response | ||||
|---|---|---|---|---|
| Variables | First CD19 CAR-T | Second CD22 CAR-T | ||
| No. (n = 27) | % of patients | No. (n = 21) | % of patients | |
| Grade of CRS | ||||
| 0 | 4 | 15 | 10 | 48 |
| 1 | 3 | 11 | 8 | 38 |
| 2 | 13 | 48 | 3 | 14 |
| 3 | 5 | 19 | 0 | 0 |
| 4 | 1 | 4 | 0 | 0 |
| 5 | 1 | 4 | 0 | 0 |
| Neurotoxicity | ||||
| Grade 1 | 1 | 4 | 0 | 0 |
| ≥Grade 2 | 2d | 7 | 0 | 0 |
| Treatment response on D30 | ||||
| CRe | 23 | 85 | 20 | 95 |
| PR | 3 | 11 | 0 | 0 |
| PD | 0 | 0 | 1 | 5 |
| Death | 1 | 4 | 0 | 0 |
- Abbreviations: BM, bone marrow; CNSL, central nervous system leukemia; CR, complete remission; CRS, cytokine release syndrome; CT, chemotherapy; DLI, donor lymphocyte infusion; EMD, extramedullary disease; HCT, hematopoietic cell transplantation; HID, haploidentical donor; HSC, hematopoietic stem cell; IFN, interferon; MSD, matched sibling donor; MUD, matched unrelated donor PD, progressive disease; PR, partial remission; TKI, tyrosine kinase inhibitor; UCB, unrelated cord blood.
- a Disease status was no longer evaluated in most patients before CD19 CAR-T cell infusion because all infusions were performed within a month (78% ≤15 days) since enrollment; and debulking chemotherapy was short-term and non-intensive, in a few cases, it was used together with lymphodepleting agents (Table S5).
- b Involved sites of EMD included central nervous system (CNS), lymph nodes, bones, soft masses, breast, mediastinum, pharynx and kidney.
- c Multiple sites were defined as ≥2 sites of EMD.
- d One case was grade 2 and 1 was grade 3.
- e Including CR with incomplete blood count recovery.
3.2 First round of CD19 CAR-T cell therapy
Treatments and outcomes are described in Table 1(B) and Table S2. Twenty-seven patients accepted the first round of CD19 CAR-T cells, with a median cell dose of 1.0 × 105/kg (range, 0.486–5.0 × 105/kg) (supplemental methods and Table S3). Since we used a fast cell culture system which only takes 5–8 days to prepare cell products, the lowest cell dose could be 0.1 × 105/kg, and patients with higher tumor burden or disease progression were given additional chemo-drugs, all 27 patients had the chance to receive CAR-T cells.
By day 30 after T-cell infusion, 23 of 27 (85%) patients achieved complete remission (including CR with incomplete blood count recovery), MRD negative CR was 95% (18/19) in evaluable cases (patients with EMD could not be evaluated for MRD); three cases (11%) with multiple EMD obtained partial remission; and one patient (4%) died of severe CRS on day 17 manifesting as pulmonary hemorrhage and multiorgan failure.
CRS occurred in 23 (85%) patients, consisting of 70% (16/23) grade I-II and 30% (7/23) severe CRS (≥grade III). All severe and 10 grade II CRS were treated by steroids (tocilizumab was not used) according to the approach we described in previous work16 and supplemental methods; four cases with severe CRS accepted additional plasma exchange. Neurologic toxicities were observed in three (11%) patients with CNSL, one patient's grade I neurotoxicity (headache) was managed only with antipyretics; another grade II neurotoxicity (headache and transient facial twitch) resolved by mannitol and intracranial dexamethasone. Patient (Pt.) 15, an adult male relapsing with isolated CNSL, had grade III neurotoxicity presenting with bilateral lower limb paralysis and dysuria. These manifestations began from 2 weeks post cell infusion and gradually progressed to a severe stage in which the patient needed urinary catheterization and was unable to walk. An MRI showed abnormal enhancement in the 3rd-5th thoracic vertebrae and spinal cord. A CNS infection was excluded by negative CSF examinations. His symptoms were obviously alleviated after 3 months and completely relieved after 6 months. Treatments included steroids, vitamins B1 and B12, monosialotetrahexosylganlioside and rehabilitation therapies.
3.3 Patients withheld from the second CAR-T cell infusion
Apart from the patient who died of CRS, another five CR patients did not receive the second CAR-T treatment due to following reasons. One died from severe extensive cGVHD at 5.5 months after CAR-T. The patient suffering from grade III neurotoxicity mentioned above was administered dasatinib (Ph + B-ALL) as maintenance therapy to avoid potential recurrent neurological damage caused by CAR-T treatment; he had been in CR for 23.7 months. Unsolved moderate cGVHD and pulmonary infection made two patients ineligible for a second CAR-T; of them, one relapsed at 10.8 months and one stayed in remission for 20.1 months. The last patient had severe Pneumocystis jirovecii pneumonia occurring at 2 months post CAR-T. He recovered after 1 month of treatment involving non-invasive ventilator support, but refused the second CAR-T, and remained in CR for 10.5 months (Figure 1(A)).
3.4 Second round of CD22 CAR-T cell therapy
Twenty-one patients received the second round of CAR-T treatment targeting CD22 antigen. The median time interval between two infusions was 2.7 (range, 1.5–6.5) months and the median dose of infused cells was 2.0 × 105/kg (range, 0.32–5.0 × 105/kg).
Before the second CAR-T cell infusion, 16 patients remained in CR, three PR cases were still in PR, and two relapsed at 1.5 and 2.2 months after first infusion (one was MRD+ and one showed hematologic relapse); 57% (12/21) of patients had detectable CD19 CAR-T cells and 18 of 19 CR/PR patients displayed B-cell aplasia. By Day 30 after the second infusion, the patient with hematologic relapse and three PR patients achieved CR, the MRD+ case had no response and progressed to hematologic relapse, and 16 CR patients remained in CR (Figure 1(A) and Table S2).
Cytokine release syndrome occurred in only 52% (11/21) of patients, all were grade I (n = 8) or grade II (n = 3), steroids were administered in three cases with grade II CRS. There was no severe CRS, neurotoxicity, or death (Table 1(B) and Figure S2(A)). The incidence and severity of CRS were much lower than those during the first CAR-T treatment when patients had higher disease burden (incidence, 52% vs. 85%, p = .013; severe CRS, 0% vs. 26%, P = .014). Among five cytokines IL-6, TNF-α, IL-10, sCD25 and IFN-γ that we routinely detected during CAR-T therapy, the mean peak values of two major ones IL-6 and sCD25 were significantly lower compared to those in first CAR-T (IL-6, p = .006; sCD25, p = .001; Figure S2(B)–(F) and Table S4). Although almost 50% of patients had no CRS, CAR-T cell expansion (Figure S3) was still observed in 95% (19/20, one had no data) of patients, and the mean peak CAR-T cell number in peripheral blood (PB) was similar to that in first CAR-T (p = .841; Figure S2(G) and Table S3). These results demonstrated that CRS and elevated serum cytokines induced by CAR-T therapy were more correlated to the interaction between tumor cells and CAR-T cells rather than CAR-T cells themselves, also supported previous observations that the severity of CRS is positively associated with disease burden.17, 18
3.5 Long-term follow-up
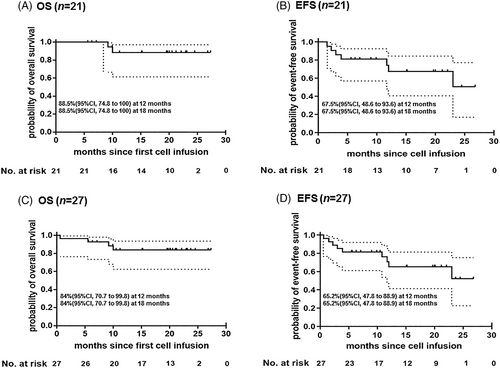
The detailed information for each patient is shown in Figure 1(B). Among the 21 patients who finished both CD19 and CD22 CAR-T cell infusions, three with BCR/ABL rearrangement continued to take TKIs, three women with breast involvement received local irradiation, one patient withdrew to undergo a second transplantation (her follow-up time was calculated until the date of transplantation), and DLI was given to one case with molecular relapse of BCR/ABL transcript. At a median follow-up of 19.7 (range, 5.6–27.3) months, 14 patients remained in CR, 2 (1 with BCR/ABL+) died of disease progression, and five relapsed with (n = 3) or without (n = 2) disease after receiving other therapies. Kaplan–Meier survival analysis showed that OS and EFS rates were 88.5% (95% confidence interval [CI], 74.8 to 100) and 67.5% (95% CI, 48.6 to 93.6), respectively, at both 12 months and 18 months (Figure 2(A), (B)). In the intention-to-treat analysis of all 27 patients (including six cases without receiving second CD22 CAR-T), OS and EFS rates were 84.0% (95% CI, 70.7 to 99.8) and 65.2% (95% CI, 47.8 to 88.9), respectively, at both 12 and 18 months (Figure 2(C), (D)).
The flow cytometry results showed that, in most patients (15/20, 75%, one had no data), CD22 CAR-T cells could not be detected within 60 days after second infusion; whereas half of patients (14/27, 52%) had detectable CD19 CAR-T cells on day 60 after CD19 CAR-T, this difference might be related to the different numbers of CD19/CD22 antigens before cell infusion. However, the median duration of B-cell aplasia (another marker of CAR-T cell persistence, defined as <3% CD19/CD22 positive lymphocytes in BM) was 9.9 (range, 2–24.5) months since first infusion in 19 of 21 patients who accepted both CD19 and CD 22 CAR-T cells (two relapsing shortly after CD19 CAR-T were excluded).
Patients with multiple EMD still had a poor prognosis. After the first CAR-T, three out of five cases (60%) could not obtain CR (two CR and three PR). Although three PR patients achieved CR after the second CAR-T, eventually, three of five patients relapsed and only two (40%) maintained CR. In five patients with CNSL who had leukemic cells in CSF but no intracranial lesions, one died of CRS, one relapsed with CNSL again and another three patients remained in CR for 15.1–23.7 months. Neurotoxicity was more often seen in these patients (3/5, 60%) (Table S6).
Among seven relapsed patients who accepted CD19/CD22 CAR-T cells, two were CD19-negative, four were CD19-positive, and one could not be evaluated because she relapsed with BCR/ABL+ only (FCM-) and remained in MRD-CR after being administered DLI and ponatinib. Patient 20 showed CD19+ expression at her first relapse at 2.2-month post CD19 CAR-T; after receiving CD22 CAR-T cells, she obtained CR but relapsed again at 2.5-month, interestingly, her blast cells turned CD19 negative and still exhibited CD19- 1 month later after failing other salvage therapies (Figure S1(B)). As for CD22 antigen, 5/6 patients showed normal expression (NE) and 1/6 showed partial expression (PE) (NE and PE were defined as >80% and 20–80% of positive blasts, respectively). Cell density of CD22 was not assessed by the Fry et al method.10
3.6 CAR-T associated GVHD
In these post-HCT patients whose donors were mainly (74%) haplo-identical family members, GVHD post CAR-T therapy emerged as another adverse effect, occurring after both first and second CAR-T. The total incidence of CAR-T associated GVHD was 23% (6/26, except for one died of CRS on day 17), with 8% (2/26) aGVHD and 15% (4/26) cGVHD related to preexisting cGVHD before CAR-T therapy. We compared the difference between patients with and without GVHD in the mean numbers of infused CAR-T cells and expanded peak CAR-T cells; the mean values of peak cytokines; and the percentages of patients with preexisting GVHD before CAR-T, receiving haplo-identical transplantation and recent DLI (Table S7), the results showed that preexisting GVHD (p = .001) and recent DLI (p = .046) were related to post CAR-T GVHD.
The information of six involved patients is shown in Table 2. Two patients with no previous GVHD history experienced grade II aGVHD, the onset time was 1.5 and 2 months after CAR-T. One (Pt.25) with gut GVHD recovered in 40 days by taking steroids; one (Pt.13) presenting with skin GVHD and elevated aminotransferases developed cGVHD, steroids were intermittently administered due to recurrent pulmonary infections (bacteria and fungus). Among four patients having mild cGVHD before CAR-T, 2 (Pt.4 and 11) with oral and/or skin GVHD presented the similar or slightly worse manifestation (still in mild stage) after both infusions. Pt.11 had not taken any anti-GVHD medication, Pt.4 was treated by topical steroids (budesonide suspension for oral cavity) and ruxolitinib for short term; their symptoms were completely resolved 1 year after the first CAR-T. However, the other two patients (Pt.7 and 9) with liver and skin cGVHD, who experienced grade III aGVHD induced by DLI and subsequent cGVHD prior to enrollment, got worse after the first CAR-T (therefore without undertaking the second infusion). Patient 9 developed moderate cGVHD which persisted after treatment with steroids and tacrolimus, and complicated by concurrent pulmonary aspergillus infection. Patient 7 gradually progressed to severe extensive cGVHD, failed multiple anti-GVHD drugs including steroids, methotrexate, tacrolimus, mycophenolate mofetil and vindesine, eventually died of liver failure at 5.5 months after CAR-T.
| Pt. No. | Type of transplant | GVHD status | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Recent GVHD before enrollment | At enrollment before CAR-T | After first CAR-T | After second CAR-T | Treatment | BCA (months) (till relapse or last follow-up) | Disease status | GVHD | ||
| 4 | MSD | cGVHD (Mild, mouth) | cGVHD (Mild, mouth) | cGVHD (Mild, mouth) | cGVHD (Mild, mouth) | Topical steroids (budesonide suspension for oral cavity) and ruxolitinib | 23 | Relapsed at 23 months | Recovered in 1 year |
| 7 | HID | aGVHD (III, skin and gut) induced by DLI | cGVHD (Mild, liver and skin) | cGVHD (Severe, extensive) | N/A | Steroids, methotrexate, tacrolimus, mycophenolate mofetil and vindesine | 5.5 | Died of GVHD at 5.5 months | Persisted |
| 9 | HID | aGVHD (III, skin and gut) induced by DLI | cGVHD (Mild, skin and liver) | cGVHD (Moderate, liver and skin) | N/A | Steroids and tacrolimus | 10.8 | Relapsed at 10.8 months | Persisted |
| 11 | HID | cGVHD (Mild, skin) | cGVHD (Mild, skin) | cGVHD (Mild, skin and mouth) | cGVHD (Mild, skin and mouth) | - | 24.5 | CCR | Recovered in 1 year |
| 13 | HID | — | — | aGVHD (II, skin)+cGVHD(Moderate, lung and skin) | N/A | Steroids | 20.1 | CCR | Persisted |
| 25 | HID | — | — | — | aGVHD (II, gut) | Steroids | 15.1 | CCR | Recovered in 40 days |
- Abbreviations: aGVHD, acute GVDH; BCA, B-cell aplasia; CCR, continuous complete remission; cGVHD, chronic GVHD; DLI, donor lymphocyte infusion; GVHD, graft versus host disease; HID, haploidentical donor; MSD, matched sibling donor; Pt. No., patient number.
4 DISCUSSION
Although CD19 CAR-T therapy has a dramatic effect on r/r B-ALL patients with high CR rates of 81–90%,5-9 around 20–50% of patients relapsed at 6 months.5, 7, 9 To overcome the drawback of single-target CAR-T treatment, the combination of both CD19 and CD22 CAR-T therapy has been conducted by a few groups, which included bi-specific CD19/CD22 CARs and cocktail CD19/CD22 regimens.19-21 In this study, we sequentially applied CD19 and CD22 CAR-T cells to treat post-transplant relapsed B-ALL patients with poor prognosis.
The first round of single CD19 CAR-T treatment resulted in a higher CR rate of 85% (23/27). Subsequently, among 21 patients who finished both CD19 and CD22 CAR-T therapies, the OS and EFS rates were 88.5 and 67.5%, respectively, at both 12 months and 18 months. In comparison with our previous outcomes of single CD19 or CD22 CAR-T therapy for r/r B-ALL, in which 50–57% of patients who did not undergo transplantation post CAR-T relapsed within 6–8 months,8, 11 this new strategy remarkably improved treatment outcomes. Although our results could not be directly compared to other reports, data here showed higher EFS and OS rates at 12–18 months; more importantly, this occurred in post-HCT patients among whom more than 50% were adults.
We have previously verified that shot-term steroids used to treat CRS do not influence the efficacy and kinetics of CAR-T cells in B-ALL,16 same results were seen here (Figure 1(B) and Figure S3). Additionally, in five patients with CART-associated GVHD who received a longer course of steroids or combined with other immunosuppressive medications, B-cell aplasia and treatment outcomes were not affected either (Table 2).
The necessity of a second CAR-T treatment for PR or relapsed patients was undoubtable. Under the circumstance of CR, we hypothesize that the second CAR-T could eradicate the MRD below the threshold of current assays or hidden in extramedullary sites. The leukemic blasts of patient 20 showed CD19 positivity at her first relapse after CD19 CAR-T while her CAR-T cell number in PB was still higher at 2.45 × 107/L, implying that her CD19 CAR-T cells lost the function of killing leukemic blasts. After CD22 CAR-T cell infusion, she obtained MRD-CR but relapsed again; unexpectedly, this time, her blasts exhibited CD19 negativity and remained CD19-negative 1 month later (no further data since she was discharged and died). These data provided evidence for another possibility that a second CAR-T cell treatment might strengthen the function and prolong the persistence of the first CAR-T cells, the underlying mechanism remains unknown.
One concern about the effectiveness of second CD22 CAR-T therapy in CR patients is that the patients might not have CD22 antigens to drive CAR-T cell expansion. Among our 16 patients who remained in CR before CD22 CAR-T cell infusion, four had less than 1% normal CD22+ B-cells and 12 had no detectable B-cells in BM. Nevertheless, after the second infusion, CD22 CAR-T cells were detectable in 14/16 (one had no data) patients, five of whom had high peak numbers >1 × 108/L. These results revealed that CR patients with very low or no CD22 expression in BM had CAR-T cell proliferation as well, we assumed that this cell expansion could be triggered by antigens in tissues instead of those in BM, or by very few antigens that are undetectable by FCM in BM.
There was little published data on CAR-T associated GVHD in post-transplant patients, and available studies showed controversial results.22-25 In this study, CAR-T associated GVHD occurred in 23% of patients, with both new-onset aGVHD (8%) and persistent or worsened pre-existing cGVHD (15%). Several risk factors might attribute to the higher incidence of GVHD in this cohort of patients: (1) four (15%) patients had preexisting mild cGVHD before CAR-T; (2) most patients (74%) underwent HLA-haploidentical instead of HLA-matched transplantation; (3) the co-stimulator of our CARs was 4-1-BB rather than CD28, it has been demonstrated that CD28-costimulated CD19-CARs significantly decreased GVHD.26 Based on our data here that preexisting oral/skin cGVHD was not aggravated whereas liver cGVHD (induced by DLI) was remarkably exacerbated after CAR-T cell infusion, we suggest that patients with liver cGVHD prefer not to choose CAR-T therapy and DLI before CAR-T had better be avoided; whereas patients with oral/skin cGVHD are eligible for CAR-T treatment. Additionally, CAR modification of T-cells such as allodepleted T-cells, virus-specific T-cells, or natural-killer T-cells may help to reduce the risk of GVHD.27, 28
We also analyzed treatment outcomes of patients with EMD. In six cases with single-site involvement, five (83%) obtained CR after the first T-cell infusion (one died of CRS) and all remained in CR (including one without receiving the second CAR-T due to severe neurotoxicity). However, the efficiency of CAR-T cells on multifocal EMD was not encouraging, among five patients with multiple EMD, only two (40%) achieved CR following the first T-cell infusion; although another three PR patients achieved CR after second CAR-T, three of five cases relapsed. These results indicated that relapsed B-ALL patients with single EMD had a much better outcome than those with multifocal EMD, the latter requires other approaches such as CAR-T combined with anti-PD1 antibody or second transplantation to improve prognosis.
In conclusion, our sequential combination strategy of CD19 and CD22 CAR-T therapies achieved longer EFS and OS in relapsed post-transplant B-ALL patients, which was beneficial to patients with single-site EMD as well.
ACKNOWLEDGMENTS
We thank all medical staff and patients who participated in the trial, and Mr. Wei Han and Mr. Hongwei Yu from Beijing eStartMed company for statistical analysis. This work was supported by Creative Research Project of Gaobo Healthcare Group (No. GBHG-CR2017003); and the National Key Basic Research Program of China (No. 2016YFC1303403).
CONFLICT OF INTEREST
Alex H. Chang is also a founding member of Shanghai YaKe Biotechnology Ltd., a biotechnology company focused on research and development of tumor cellular immunotherapy. The remaining authors declare no conflict of interest.
This study was approved by Beijing Boren Hospital institutional review board and registered on the Chinese Clinical Trial Registry/WHO International Clinical Trial Registry (ClinicalTrials#: ChiCTR-ONC-17013648), written informed consents were obtained in accordance with the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
Data in article are available in supplementary materials. For original data, please contact Zhichao Yin by e-mail at [email protected] or corresponding authors.




